🧪 Researchers explore the influence of divalent cations, specifically Ca2+ and Mg2+, on the phase behavior of elastin-like polypeptides (ELPs), crucial in understanding macromolecular conformation changes. The study investigates the molecular mechanisms behind salting-out and salting-in phenomena exhibited by ELPs in response to different divalent salts.
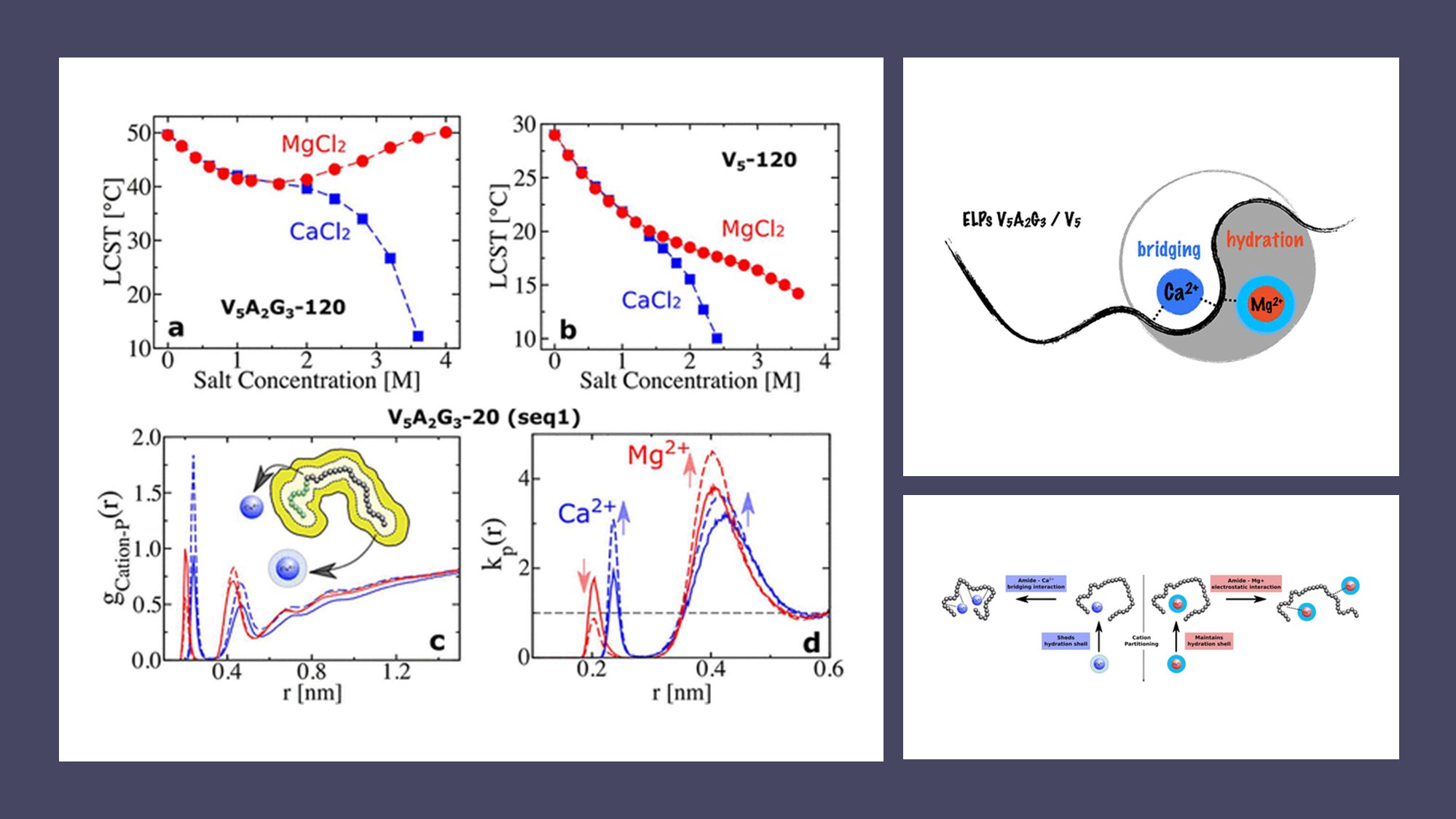
- 🌐 Salting-out and salting-in behaviors of ELPs in divalent cation solutions are observed, with Ca2+ inducing salting-out and Mg2+ showing both salting-out and salting-in effects at varying concentrations.
- 🧬 Two ELP sequences, V5-120 and V5A2G3-120, are studied, highlighting sequence-dependent phase behaviors.
- 🤿 Molecular dynamics simulations reveal that Ca2+ ions form ion bridges with amide oxygens, leading to rapid salting-out, while Mg2+ ions, tightly bound to hydration waters, cause salting-in at higher concentrations.
- 🔬 Triglycine motifs in ELPs play a significant role in regulating phase behavior, influencing the interaction between divalent cations and the polymer chain.
- 🎯 Understanding these interactions is crucial for intrinsically disordered proteins (IDPs) linked to neurodegenerative diseases, shedding light on potential accelerators or inhibitors of neurotoxic aggregates.
- 📊 Experimental validation using infrared spectroscopy confirms cation–amide oxygen interactions, emphasizing the relevance of molecular dynamics simulations.
- 🔄 The study highlights a competition between cation–amide oxygen electrostatic interactions and cation hydration strength as the determining factor in ELPs’ phase behavior.
In this molecular dynamics study, researchers delve into the intricate interplay between divalent cations (Ca2+ and Mg2+) and elastin-like polypeptides (ELPs), specifically V5-120 and V5A2G3-120 sequences. The investigation reveals a nuanced phase behavior influenced by the type and concentration of divalent salts. While Ca2+ induces salting-out by forming ion bridges with amide oxygens, leading to a swift decrease in cloud point temperature, Mg2+ exhibits a dual role—salting-out at lower concentrations and salting-in at higher concentrations due to its tight binding with hydration waters. Triglycine motifs in ELPs emerge as key regulators, influencing the interaction between cations and the polymer chain. The study’s implications extend to intrinsically disordered proteins (IDPs), especially those linked to neurodegenerative diseases, where understanding macromolecular conformation changes is critical. Experimental validation through infrared spectroscopy reinforces the findings, emphasizing the significance of molecular dynamics simulations in unraveling the complex dynamics of macromolecular systems.
Dive into the world of Elastin-like Polypeptides! 🧪 Researchers @SwamiBharadwaj @VegtLab@TUDarmstadt @penn_state @Halil_I_OKUR
— RJ (@researchersjob) November 10, 2023
@ShivNadarUnivexplores their solvation behavior in divalent metal salt solutions, in biochemistry and material science. https://t.co/Y9sSuKhQ50 pic.twitter.com/o0F5l9xic6
The researchers emphasize the importance of their study in providing atomistic insights into cation–IDP interactions, focusing on a model system of elastin-like polypeptides. They highlight the competition between cation–amide oxygen electrostatic interactions and cation hydration strength as the main determinant of ELPs’ phase behavior. The role of triglycine motifs in regulating this behavior is underscored, offering valuable insights into the impact of divalent cations on macromolecular conformations.
In a study published in the Journal of Physical Chemistry Letters, scientists have delved into the intricate dance of ions, specifically divalent cations like Ca2+ and Mg2+, and their profound impact on the behavior of macromolecules in aqueous solutions.
The researchers focused on elastin-like polypeptides (ELPs), which exhibit a fascinating lower critical solution temperature (LCST) transition, leading to expanded–collapsed conformational changes in response to varying salt concentrations. The salts in question, namely Ca2+ and Mg2+, were found to wield significant influence over the phase behavior of ELPs.
Surprisingly, the team discovered that the interaction between Mg2+ and the amide oxygen of ELPs could result in either salting-out or salting-in behavior, contingent upon the specific ELP sequence. On the other hand, Ca2+ consistently induced salting-out behavior for both ELP sequences studied.
Notably, the presence of triglycine motifs in ELPs emerged as a key player in regulating their phase behavior in salt solutions. Molecular dynamics simulations uncovered a fierce competition between cation–amide oxygen and cation–hydration water interactions, determining the ultimate effect of divalent salts on ELPs.
This research not only deepens our understanding of the complex interplay between ions and macromolecules but also sheds light on potential applications, especially in the context of intrinsically disordered proteins (IDPs). Given that the aggregation of IDPs is implicated in neurodegenerative diseases, unraveling the atomistic details of cation–macromolecule interactions opens avenues for therapeutic interventions targeting these debilitating conditions.
Solvation Behavior of Elastin-like Polypeptides in Divalent Metal Salt Solutions
- Yani Zhao, Swaminath Bharadwaj, Ryan L. Myers, Halil I. Okur, Pho T. Bui, Mengrui Cao, Lauren K. Welsh, Tinglu Yang, Paul S. Cremer, and Nico F. A. van der Vegt
- ELPs in Metal Solutions
- Experimental and Simulation Insights
- International Research Collaboration
- Biochemistry and Material Science
- 🧬 ELPs examined in divalent metal salt environments
- 🌊 Solvation characteristics explored through experiments and simulations
- 🌐 Detailed experimental and simulation methodologies provided in supporting information
- 🌱 Research collaboration involves institutions in Germany, India, and the USA
- 🧪 Potential applications in biochemistry and material science
- 🌐 Contact information for the lead researchers provided in the publication
- 📚 Supporting information includes experimental and simulation details