🔬 Researchers have developed an innovative iridium catalyst with a unique ligand framework, enabling the remote meta selective borylation of aromatic compounds, including phenols and indole derivatives. This breakthrough addresses a longstanding challenge in chemical synthesis and has broad implications for organic chemistry and the pharmaceutical industry.
🔬 Researchers @buddhadeb1979 @ChattopadhyayL1 achieve remote meta selective #borylation of aromatic compounds, addressing a long-standing challenge in chemical synthesis. Promising implications for #drug development and organic chemistry. https://t.co/55NUerqFLk pic.twitter.com/PunkUMil9U
— RJ (@researchersjob) November 2, 2023
🧪 Challenging Problem: Remote meta selective C–H functionalization of aromatic compounds is a difficult problem in chemical synthesis.
🔑 Key Factors: Success in metal catalysis for C-H bond activation and functionalization relies on two key factors: (i) designing highly reactive catalyst systems through innovative ligand frameworks and (ii) modifying substrate structures to control site selectivity.
📚 Innovative Approaches: Recent approaches have been developed to functionalize proximal and remote C–H bonds of arenes using new ligand frameworks, templates, transient mediators, and directing groups.
🌿 Importance of Phenols: Phenols, a widespread class of aromatic compounds, have diverse applications in bioactive molecules, drugs, biopolymers, and industrial processes, making their direct functionalization highly desirable.
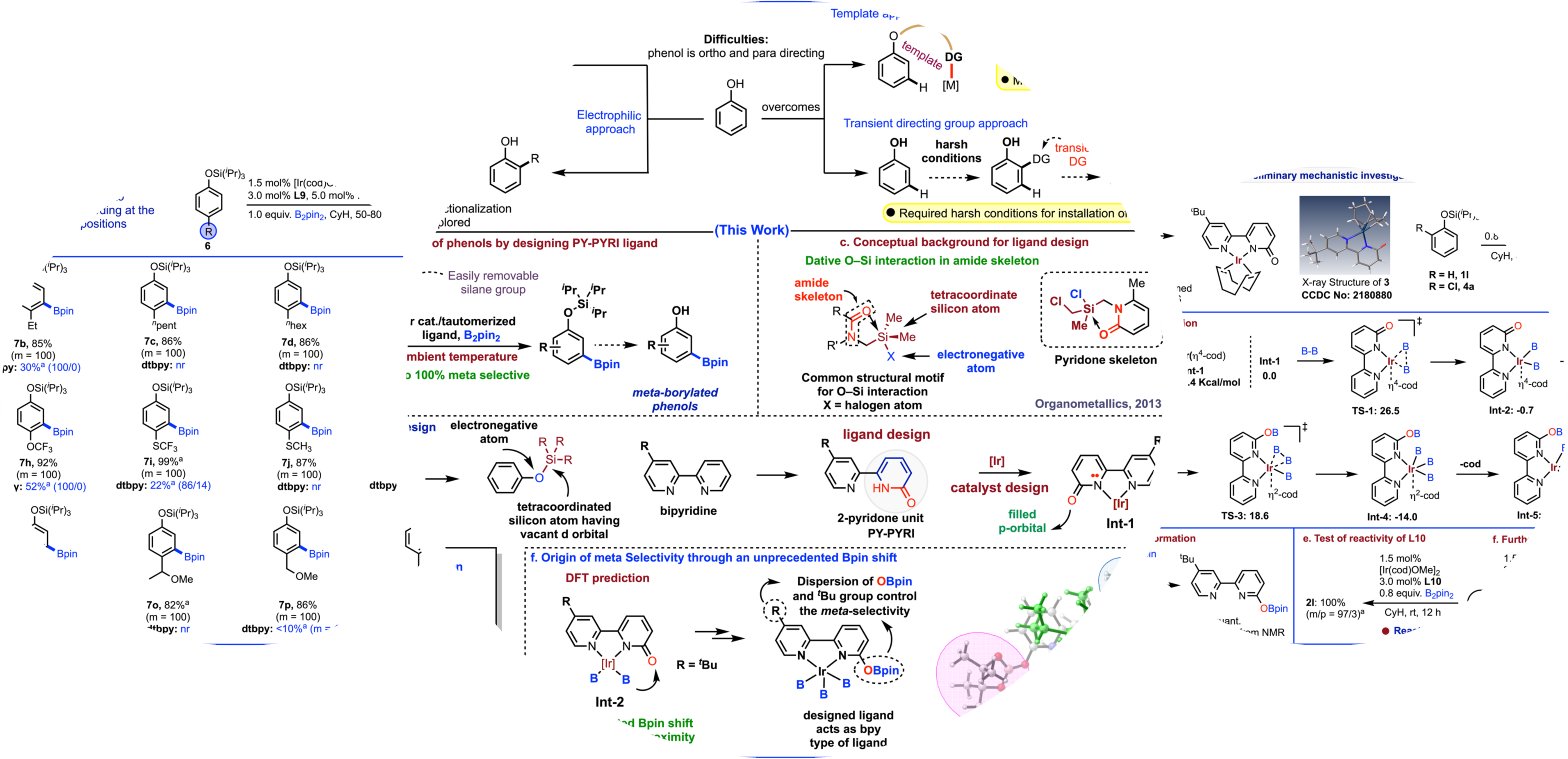
🧪 Catalytic Concept: The researchers introduced a novel ligand design strategy for the meta selective C-H borylation of phenols, inspired by weak O–Si interactions.
💡 Unprecedented Bpin Shift: Computational investigations revealed that an unprecedented Bpin shift was critical for controlling remote meta selectivity.
Remote meta selective borylation of aromatic compounds, especially phenols, has been a challenging task in the field of chemical synthesis. However, a groundbreaking approach has been developed using an iridium catalyst with a unique ligand framework that introduces a boron functionality at the remote meta position of aromatic compounds. This achievement is significant due to the broad applications of phenols in the production of bioactive molecules, drugs, and various industrial products. The inspiration for this innovative catalytic concept came from the intriguing O–Si secondary interaction, which was further explored through detailed computational investigations. The researchers discovered that an unprecedented Bpin shift was the key to controlling remote meta selectivity, enabling the efficient borylation of phenols and indole derivatives. This breakthrough has the potential to revolutionize the field of organic synthesis and significantly impact the pharmaceutical industry.
A groundbreaking study reports a highly efficient method for meta-selective C-H borylation of phenols, a challenging process in chemical synthesis. This innovative approach relies on an iridium catalyst with a specially designed ligand that facilitates remote meta-borylation, expanding the possibilities in drug molecule synthesis.
Facts
- Challenging Meta-Selective C-H Borylation: Achieving meta-selective C-H borylation of phenols, particularly in bioactive and drug molecules, has historically been a significant challenge in chemical synthesis.
- Innovative Catalyst: The study introduces a novel iridium catalyst with a bidentate pyridine-pyridone (PY-PYRI) ligand framework, which proves highly effective in facilitating meta-selective borylation reactions.
- Inspired by O-Si Interaction: The catalyst’s design concept draws inspiration from the O-Si secondary interaction, allowing for the control of remote meta selectivity through dispersion between the ligand and the steering silane group.
- Optimization of Reaction Conditions: The study optimizes reaction conditions by identifying the most suitable steering group (SiiPr3) for meta-borylation of phenols.
- Ligand Design Impact: Various ligand designs were explored, with ligand L9 (PY-PYRI) showing remarkable performance in achieving meta selectivity and excellent conversion.
- Catalyst Stability: The catalyst (L9) proved to be highly stable, even when stored in open air for several months.
- Expanded Substrate Scope: The developed catalyst and reaction conditions were successfully applied to a wide range of phenols with different substituents, resulting in high meta selectivity and yields.
This groundbreaking research addresses a long-standing challenge in chemical synthesis, the meta-selective C-H borylation of phenols. By developing a unique iridium catalyst with a specially designed ligand (PY-PYRI), the study enables efficient meta-borylation reactions even in the most remote meta positions of phenols, including those found in bioactive and drug molecules. The catalyst’s design draws inspiration from the O-Si secondary interaction, allowing for the control of remote meta selectivity through dispersion between the ligand and the steering silane group. Through careful optimization of reaction conditions, including the choice of steering group and ligand design, the study achieves exceptional meta selectivity and conversion rates. The resulting catalyst proves to be highly stable, making it a valuable tool for the rapid synthesis of essential compounds in various fields. Furthermore, the methodology was successfully applied to a wide range of phenols, demonstrating its versatility and potential impact on the field of chemical synthesis.
What Researchers of the Paper Say
The researchers emphasize the significance of their work in addressing the long-standing challenge of achieving meta-selective C-H borylation in phenols, which has been particularly problematic for bioactive and drug molecules. They highlight the unique design of the iridium catalyst, which draws inspiration from the O-Si secondary interaction and dispersion forces to control remote meta selectivity. The researchers underscore the remarkable performance of the developed ligand (PY-PYRI) and its impact on achieving high meta selectivity and excellent conversion rates. They also stress the stability of the catalyst, making it a practical tool for various applications. Moreover, the researchers emphasize the broad applicability of their methodology, as it was successfully extended to a wide range of phenols with different substituents, offering new possibilities in chemical synthesis.
A groundbreaking study published in Nature Communications has unveiled a revolutionary method for precise chemical modification of bioactive and drug molecules. The research, titled “Efficient Meta-Selective C-H Borylation of Phenols Enabled by a Unique Iridium Catalyst,” showcases the development of an innovative iridium catalyst with a bidentate pyridine-pyridone (PY-PYRI) ligand framework. This catalyst allows for the highly efficient meta-selective C-H borylation of phenols, a challenging process in chemical synthesis.
Traditionally, achieving meta-selective C-H borylation in phenols has proven exceedingly difficult due to the extreme inertness of meta C-H bonds. This groundbreaking work introduces a new design concept inspired by the O-Si secondary interaction, enabling control of remote meta selectivity through dispersion forces between the designed ligand and the steering silane group. The study optimizes reaction conditions, identifies the most suitable steering group (SiiPr3), and explores various ligand designs. The result is a remarkably stable catalyst that can be stored in open air for extended periods.
The impact of this research extends beyond theory, as it was successfully applied to a wide range of phenols, demonstrating its versatility and practicality in the field of chemical synthesis. The methodology opens new possibilities for the precise modification of bioactive molecules, drug synthesis, and various industrial applications. This breakthrough promises to reshape the landscape of chemical synthesis and offer innovative solutions for the development of essential compounds in the pharmaceutical and chemical industries.
A tautomerized ligand enabled meta selective C–H borylation of phenol
- Saikat Guria, Mirja Md Mahamudul Hassan, Jiawei Ma, Sayan Dey, Yong Liang & Buddhadeb Chattopadhyay
- Remote meta selective borylation of aromatic compounds is a challenging problem in chemical synthesis.
- Innovative ligand design with iridium catalysts enables the selective introduction of boron functionalities in remote meta positions.
- Phenols, widely used in the pharmaceutical and industrial sectors, can now be efficiently functionalized through this method.
- The catalytic concept was inspired by O–Si interactions and an unprecedented Bpin shift.
- This breakthrough has the potential to revolutionize organic synthesis and impact the pharmaceutical industry.