New Study Shows Innovative Material Design Concept for Water-Stable and Photoconductive One-Dimensional Hybrid Lead Bromide
Outcomes:
- Scientists have developed a new material design concept for preparing low-dimensional hybrid perovskite structures that exhibit impressive optoelectronic activity and water stability.
- The combination of an intermolecular noncovalent (cation−π) interaction and intramolecular π-conjugation is the key to this innovative material design concept.
- The study results provide a new approach to creating water-stable and photoconductive materials.
Scientists have developed a new material design concept for preparing low-dimensional hybrid perovskite structures that exhibit impressive optoelectronic activity and water stability. The combination of an intermolecular noncovalent (cation−π) interaction and intramolecular π-conjugation is the key to this innovative material design concept. The study results provide a new approach to creating water-stable and photoconductive materials. The research was published in the Journal of Physical Chemistry Letters.
Details:
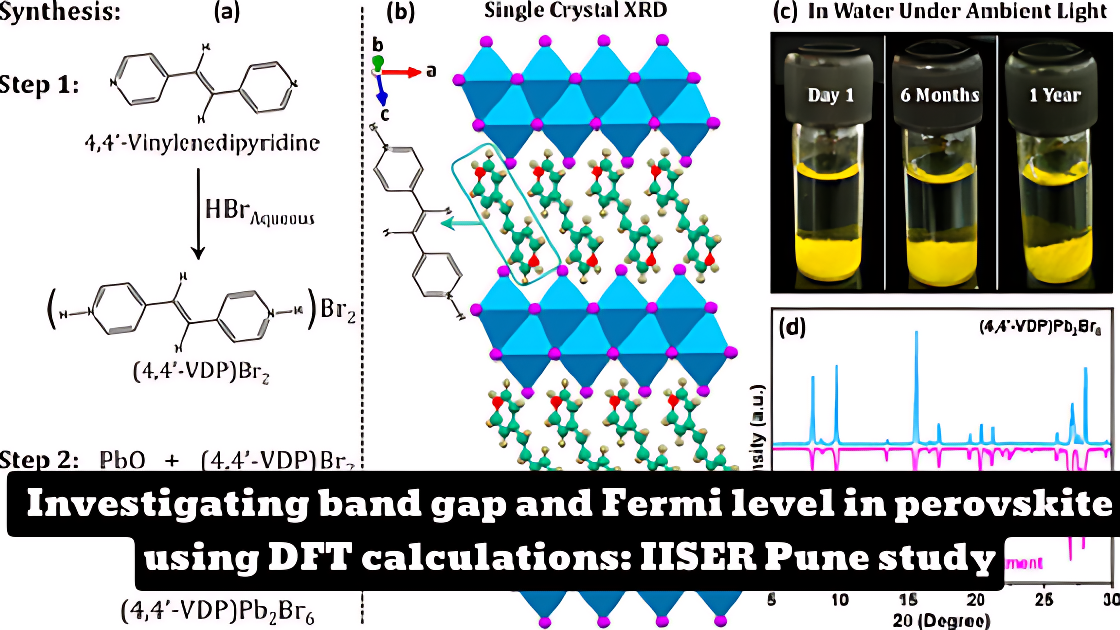
- The study reports the development of water-stable and photoconductive (4,4′-VDP)Pb2Br6 single crystals with a 1D perovskite derivative structure.
- The results show that the long-range intermolecular cation−π interaction in the organic sublattice yields completely (more than a year) water-stable crystals.
- The 4,4′-VDP molecular ion has π-conjugation throughout the molecule, which allows the 1D Pb–Br units to electronically interact with each other via the delocalized π-electrons of the organic sublattice.
- This interaction shifts the total DOS (and projected DOS) toward the Fermi level, reducing the band gap and significantly improving the photoconductivity.
- For comparison, the study also prepared single crystals of (4,4′-EDP)Pb2Br6, which has the same crystal structure and a similar composition compared to (4,4′-VDP)Pb2Br6, but (4,4′-EDP)Pb2Br6 does not have π-conjugation between the two pyridinium rings and therefore exhibits an ∼0.85 eV wider band gap and no photocurrent, in sharp contrast to (4,4′-VDP)Pb2Br6 crystals.
Combining π-Conjugation and Cation−π Interaction for Water- Stable and Photoconductive One-Dimensional Hybrid Lead Bromide
Sheikh; Anilkumar; Das; Rahman; Chakraborty; Nag 2023. 2023
Full-text link: https://doi.org/10.1021/acs.jpclett.2c03861
What this paper is about
- The long-range cation interaction between the ammonium ion of one 4,4-EDP cation and the -electron cloud of the adjacent cation dominates over their interaction with water molecules.
- However, the crystal structure of Pb 2 Br 6 has a one-dimensional network of a Pb 2 Br 6 sublattice, as shown by horizontal red-colored lines in.
- Similar water stability was also reported for a zero-dimensional hybrid CuI system, by employing interactions between organic cations, but charge transport is also inhibited in that sample.
What you can learn
The results are similar to the recently reported data for [ 2 NH2 NH 3.
- The optical absorption and photoconductivity measurements, in combination with the DFT calculations, show that conjugation in the organic sublattice can electronically connect the adjacent 1D semiconducting inorganic frameworks of Pb 2 Br.
- For comparison, we also prepared single crystals of Pb 2 Br, which has the same crystal structure and a similar composition compared to Pb 2 Br, but Pb 2 Br 6 does not have conjugation between the two pyridinium rings and therefore exhibits an 0.85 eV wider band gap and no photocurrent, in sharp contrast to Pb 2 Br 6 crystals.
What is the focus of the paper “Combining π-Conjugation and Cation−π Interaction for Water- Stable and Photoconductive One-Dimensional Hybrid Lead Bromide”?
The paper focuses on designing low-dimensional hybrid perovskite structures that are water-stable and photoconductive through the combination of π-conjugation and cation-π interaction.
How does the crystal structure of Pb 2 Br 6 differ from that of the zero-dimensional hybrid CuI system?
The crystal structure of Pb 2 Br 6 has a one-dimensional network of a Pb 2 Br 6 sublattice, while the zero-dimensional hybrid CuI system employs interactions between organic cations.
What is the significance of the intermolecular cation-π interaction in the organic sublattice?
The intermolecular cation-π interaction in the organic sublattice yields completely water-stable crystals and contributes to the impressive optoelectronic activity of the perovskite structures.
How does the conjugation in the organic sublattice of Pb 2 Br 6 crystals affect their optoelectronic properties?
The conjugation in the organic sublattice of Pb 2 Br 6 crystals electronically connects the adjacent 1D semiconducting inorganic frameworks of Pb 2 Br, reducing the band gap and significantly improving the photoconductivity.
What is the difference between the optoelectronic properties of (4,4′-VDP)Pb2Br6 and (4,4′-EDP)Pb2Br6 crystals?
(4,4′-VDP)Pb2Br6 crystals have π-conjugation between the two pyridinium rings, resulting in a narrower band gap and improved photoconductivity, while (4,4′-EDP)Pb2Br6 crystals lack this conjugation and have a wider band gap and no photocurrent.
Basics Q&A related to this research
What is photoconductive?
Photoconductive refers to the ability of a material to conduct electricity when it is exposed to light. In other words, a photoconductive material can change its electrical properties in response to light.
What is hybrid?
Hybrid refers to something that is made by combining two or more different elements or components. In the context of materials science, a hybrid material is one that is composed of two or more different types of materials, such as organic and inorganic components.
What is crystal structure?
Crystal structure refers to the arrangement of atoms, ions, or molecules in a crystal. The crystal structure determines the physical properties of the crystal, such as its shape, size, and optical properties.
What is water stability?
Water stability refers to the ability of a material to maintain its structure and properties when it comes into contact with water. A water-stable material is one that does not degrade or dissolve in the presence of water.
What is cation-π interaction?
Cation-π interaction refers to the attractive forces between a positively charged ion (cation) and a nearby π electron cloud. This type of interaction is important in the formation of some types of materials, such as organic-inorganic hybrid materials.
What is perovskite?
Perovskite refers to a class of materials that have a specific crystal structure, which is named after the mineral perovskite. Perovskite materials have unique electronic and optical properties, which make them useful in a wide range of applications, including solar cells, LEDs, and lasers.
What is π-conjugation?
π-Conjugation refers to the arrangement of atoms in a molecule in such a way that the π electrons are delocalized, or spread out over the entire molecule. This type of arrangement gives molecules unique electronic properties, such as increased stability and enhanced light absorption.
What is optical absorption?
Optical absorption refers to the ability of a material to absorb light at a specific wavelength or range of wavelengths. This property is important in the development of materials for applications such as solar cells and LEDs.
What are DFT calculations?
DFT (Density Functional Theory) calculations are a type of computational method used to calculate the electronic structure of materials. DFT calculations are widely used in materials science to predict the electronic and optical properties of new materials.
What is band gap?
Band gap refers to the energy difference between the highest occupied energy level (valence band) and the lowest unoccupied energy level (conduction band) in a material. The band gap determines the electrical and optical properties of a material.
What is Fermi level?
Fermi level is the energy level at which there is a 50% probability of finding an electron. The Fermi level is an important concept in materials science, as it determines the electrical properties of a material, such as its conductivity and electron affinity.
What is optoelectronic?
Optoelectronic refers to the study and application of materials and devices that can convert light energy into electrical energy, or vice versa. Optoelectronic materials are important in a wide range of applications, including solar cells, LEDs, and sensors.
What is intermolecular?
Intermolecular refers to interactions or processes that occur between different molecules. This type of interaction is important in determining the properties of materials, such as their stability and reactivity.
What is delocalized?
Delocalized refers to the spread of electrons over a large region, rather than being