This research article discusses a novel strategy for synthesizing rotaxanes, which are interlocked molecules with a ring threaded through a linear component. The strategy involves enlarging the size of the terminal phenol group of the linear component by aromatic bromination, resembling an end-capping strategy. The enlarged group prevents dethreading from the ring, allowing for the formation of rotaxanes. The method offers advantages such as mild reaction conditions, wide substrate scope, and the potential for further functionalization of the resulting rotaxanes.
🔄 Discover the future of interlocked molecules! Scientists have introduced a groundbreaking end-capping strategy for synthesizing rotaxanes, fascinating interlocked molecules with numerous applications. By enlarging the terminal phenol group through aromatic bromination, they successfully prevent dethreading of the ring, enabling the formation of rotaxanes. This innovative approach offers multiple advantages, including mild reaction conditions, a wide range of substrates, and the potential for further functionalization. Delve into the research to witness the immense possibilities of these interlocked wonders. #RotaxaneSynthesis #Innovation #ScientificAdvancements
Summary
- 🧪 Rotaxanes are interlocked molecules with a ring threaded through a linear component.
- 🔄 Rotaxane synthesis typically involves an end-capping approach, forming a pseudorotaxane complex followed by the introduction of bulky end caps.
- 🧪 In 2007, the Chiu group introduced a unique “threading-followed-by-swelling” strategy for rotaxane synthesis.
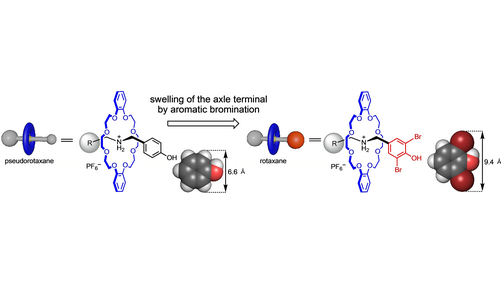
- 💡 This work presents an alternative end-capping strategy by swelling the terminal phenol group through aromatic bromination.
- 📏 The swelling size of the brominated aromatic ring is large enough to prevent dethreading, comparable to frequently used end-cap groups.
- 🔄 The present strategy combines features of both end-capping and swelling strategies, allowing for intact terminal phenolic OH groups and potential functionalization.
- 💼 The method demonstrates mild reaction conditions, wide substrate scope, and the possibility of degradative release of the axle component.
The article explores a new approach to synthesizing rotaxanes, focusing on the enlargement of the terminal phenol group of the linear component. By introducing external bromine atoms through aromatic bromination, the terminal phenol group swells in size, acting as an end cap to prevent dethreading of the ring. This strategy combines features of both end-capping and swelling approaches, allowing the intactness of the terminal phenolic OH group and offering the potential for further functionalization. This method has many benefits, including the ability to use a variety of different axle components with different swelling precursors and end groups, carrying out the reaction at room temperature with gentle conditions, and having a wide range of substrates available for use. Additionally, the brominated rotaxanes synthesized through this strategy have the potential for degradative release of the axle component under basic conditions. The experimental results indicate the effects of different organic bases and solvents on the yield of rotaxane synthesis, with DMAP catalyst and CHCl3 as the most favorable combination. The optimization of reaction conditions led to improved yields, demonstrating the efficiency and applicability of the proposed strategy.
Rotaxanes are molecules composed of a linear component threaded through a macrocycle with bulky end caps, and they have various applications in fields such as molecular machines, catalysis, sensing, and functional materials. The conventional method for rotaxane synthesis involves an end-capping approach, where a pseudorotaxane complex is formed through non-covalent interactions, followed by the introduction of bulky end caps to prevent dethreading.
In this study, researchers have proposed a new strategy for rotaxane synthesis by swelling the terminal phenol group through aromatic bromination. By enlarging the size of the terminal brominated aromatic ring, which measures 9.4 Å, the researchers aimed to prevent dethreading effectively. This strategy combines features of both end-capping and swelling strategies, offering several advantages. Firstly, it allows for the use of a variety of swelling precursors and terminal groups for the axle components. Secondly, the swelling process can be performed under mild conditions, typically at room temperature and in almost neutral conditions. Additionally, the method demonstrates a wide substrate scope, with successful synthesis of 19 examples, including the formation of a [3]rotaxane. The resulting brominated rotaxanes can be further derivatized or undergo degradative release under basic conditions by dethreading the thermally stable structures.
To optimize the new method, the researchers explored the effects of different organic bases and solvents. Organic bases such as pyridine, DABCO, NEt3, DMAP, and DBU were tested in CHCl3 as the solvent. The addition of these bases significantly improved the yields of rotaxane synthesis, but no clear correlation was observed between the pKa values of the bases and the yields. Among the bases tested, DMAP with an intermediate pKa value (9.2) resulted in the highest yield (71%).
Rotaxane Synthesis by an End-Capping Strategy via Swelling Axle-Phenols
- Koki Fujimura, Dr. Yoshihiro Ueda, Dr. Yousuke Yamaoka, Prof. Kiyosei Takasu, Prof. Takeo KawabataIndian Institute of Science Education and Research Thiruvananthapuram, India
- The size of the terminal brominated aromatic ring (9.4 Å) is large enough to prevent dethreading.
- Rotaxane synthesis was examined using different organic bases and solvents, with DMAP (4 mol % loading) yielding the highest yield (79%).