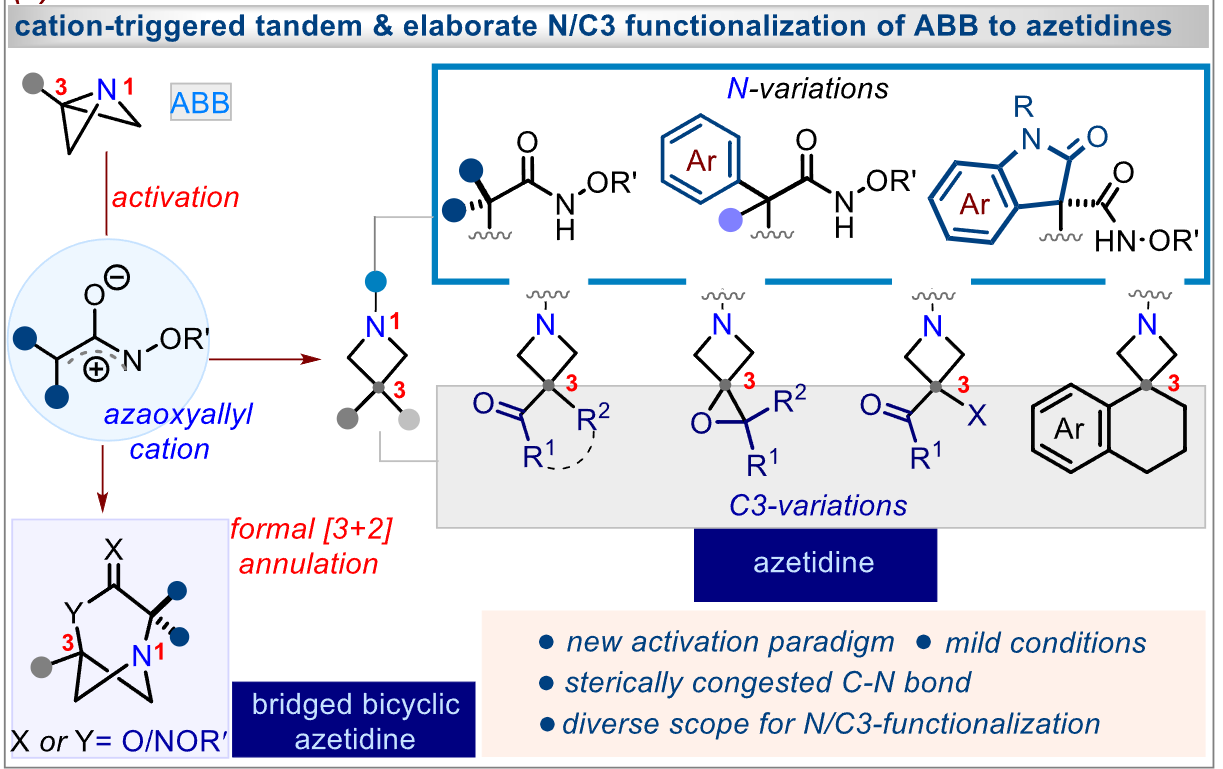
Researchers have developed a versatile cation-driven activation strategy for accessing functionalized azetidines from azabicyclo[1.1.0]butanes (ABB). This method involves N-activation and the formation of a congested C-N bond, enabling effective C3 activation. The approach holds promise for diverse applications in drug discovery and synthetic chemistry.
Facts:
- 🔬 Access to 1,3-functionalized azetidines is important for drug discovery.
- 🔬 Strain-release-driven functionalization of ABB is appealing for this purpose.
- 🔬 Previous approaches were limited to a selected set of electrophiles.
- 🔬 The new method involves cation-driven activation using Csp3-precursors.
- 🔬 N-activation leads to the formation of a congested C-N bond.
- 🔬 The approach enables effective C3 activation.
- 🔬 The concept can be extended to the synthesis of bridged bicyclic-azetidines.
Access to 1,3-functionalized azetidines is highly desirable for discovering new applications in drug discovery. The strain-release-driven functionalization of azabicyclo[1.1.0]butanes (ABB) has attracted significant interest. Previous approaches for N-activation and N-functionalization were limited to a specific group of electrophiles. In this study, researchers present a versatile cation-driven activation strategy for ABB. The method involves the use of Csp3-precursors that can form reactive (aza)oxyallyl cation in situ. The N-activation process leads to the formation of a congested C-N bond, enabling effective C3 activation. Furthermore, the concept can be expanded to achieve a formal [3+2]-annulation, resulting in the synthesis of bridged bicyclic-azetidines. The new activation paradigm offers operational simplicity and remarkable diversity, making it promising for applications in synthetic and medicinal chemistry.
Researchers have made a significant breakthrough in the field of synthetic chemistry, unveiling a versatile cation-driven activation strategy for accessing functionalized azetidines from azabicyclo[1.1.0]butanes (ABB). This innovative method, which involves N-activation and the formation of a congested C-N bond, opens up exciting possibilities for drug discovery and synthetic chemistry applications.
In this study, researchers introduce a new cation-driven activation strategy for ABB, overcoming the limitations of previous approaches. The method employs Csp3-precursors that can generate reactive (aza)oxyallyl cations in situ, leading to N-activation. This N-activation process ultimately results in the formation of a congested C-N bond, enabling effective C3 activation.
What sets this new method apart is its remarkable diversity and operational simplicity. The cation-driven activation approach offers a broader range of possibilities compared to previous methods, allowing for the synthesis of a wide variety of functionalized azetidines. Moreover, the concept can be extended to achieve a formal [3+2]-annulation, enabling the synthesis of bridged bicyclic-azetidines.
The implications of this research are significant for both synthetic chemistry and medicinal chemistry. The newfound versatility and efficiency of this cation-driven activation strategy hold great promise for accelerating drug discovery efforts. By providing access to a diverse range of functionalized azetidines, researchers can explore new chemical space and potentially identify novel therapeutic agents.
The development of this innovative method highlights the power of interdisciplinary collaboration and the ongoing advancements in synthetic chemistry. It represents a major step forward in the quest to unlock the full potential of azetidines and their derivatives.
As researchers continue to refine and expand upon this cation-driven activation strategy, the possibilities for drug discovery and synthetic chemistry are expanding exponentially. The ability to access functionalized azetidines with ease and precision brings us closer to developing groundbreaking medications and advancing the field of medicinal chemistry.
In conclusion, the cation-driven activation strategy for accessing functionalized azetidines from azabicyclo[1.1.0]butanes represents a remarkable achievement in synthetic chemistry. By harnessing the power of N-activation and the formation of a congested C-N bond, researchers have opened up new avenues for drug discovery and synthetic chemistry applications. This research paves the way for future advancements in medicinal chemistry and brings us one step closer to developing innovative and effective therapeutics.
Research Work- Cation-Promoted Strain-Release-Driven Access to Functionalized Azetidines from Azabicyclo[1.1.0]butanes
FAQ
Q: What are azetidines and azabicyclo[1.1.0]butanes?
A: Azetidines and azabicyclo[1.1.0]butanes are chemical compounds that belong to the class of saturated nitrogen-containing heterocycles. Azetidines have a four-membered ring containing three carbon atoms and one nitrogen atom, while azabicyclo[1.1.0]butanes have a bicyclic structure consisting of a three-membered ring fused with a four-membered ring, with nitrogen atoms present in both rings.
Q: What is strain-release-driven functionalization?
A: Strain-release-driven functionalization refers to a strategy used in synthetic chemistry to activate and modify chemical compounds by harnessing the energy released from the relaxation of strained molecular structures. The release of strain provides the driving force for the desired chemical transformation.
Q: What is cation-driven activation?
A: Cation-driven activation is a concept in synthetic chemistry where the presence of a cation, a positively charged ion, facilitates a chemical reaction or transformation. The cation acts as a catalyst or activator, influencing the reactivity and selectivity of the reaction.
Q: What is N-activation?
A: N-activation refers to the activation or functionalization of a nitrogen atom in a molecule. It involves modifying or transforming the chemical properties of the nitrogen atom to enable specific reactions or interactions.
Q: What is a C-N bond?
A: A C-N bond refers to a chemical bond between a carbon (C) atom and a nitrogen (N) atom. It is a covalent bond formed by the sharing of electrons between the carbon and nitrogen atoms.
Q: What is synthetic chemistry?
A: Synthetic chemistry is a branch of chemistry that focuses on the development and design of new chemical compounds through the synthesis (creation) of molecules. It involves the preparation and manipulation of organic and inorganic compounds using various chemical reactions and techniques.
Q: What is medicinal chemistry?
A: Medicinal chemistry is a field of study that combines principles of chemistry and pharmacology to discover, design, and develop new drugs. Medicinal chemists aim to identify and optimize chemical compounds with therapeutic properties, considering their effectiveness, safety, and pharmacokinetic properties for potential use as medications.