IACS Researchers have made a significant contribution to the field of electrochemistry with a study published in the Journal of the American Chemical Society. The study focuses on the impact of adding tertiary amines to the catalytic site on the FeIII/II potential and ORR catalysis in both organic solvents and aqueous solutions. By utilizing in situ SERRS-RDE, the researchers were able to uncover the intermediate stages of the complex ORR reaction process.
Bullet Point Summary:
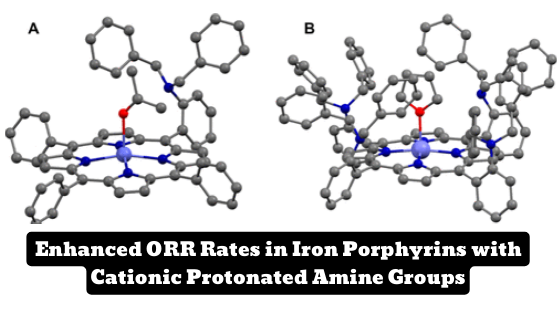
- Introduction of tertiary amine groups near catalytic site in iron porphyrins enhances ORR.
- ORR rates of FeDB and FeOB are 2 to 3 orders of magnitude higher than FeTPP.
- O2 binding rate is enhanced by at least 1 order of magnitude with positive residues in the second sphere.
- ORR rates for iron porphyrins are faster than rates based on overpotentials in both homogeneous and heterogeneous conditions.
The introduction of tertiary amine groups near the catalytic site in iron porphyrins results in significantly higher oxygen reduction rates, according to a new study.
The study, published in the Journal of the American Chemical Society, investigates the effect of introducing tertiary amines near the catalytic site on the FeIII/II potential and electrochemical oxygen reduction reaction (ORR) catalysis in both organic solvents and aqueous solutions. The authors use in situ surface-enhanced resonance Raman spectroscopy coupled to rotating-disk electrochemistry (SERRS-RDE) to detect intermediates formed during the complex reaction landscape of the ORR.
The results showed that in organic solvents, the rate of the ORR catalyzed by iron porphyrins is the protonation of the FeIII. However, the situation is different under aqueous conditions where the ORR is performed under heterogeneous conditions. The authors found that the rate enhancement of iron porphyrins with cationic protonated amine groups in the second sphere is caused by faster O2 binding and faster OO heterolysis.
The complexes FeDB and FeOB were drop-casted over an electrode, and then electrochemical data were collected under heterogeneous conditions in pH 7 phosphate buffer solution and compared with those of a previously reported complex. In an air-saturated buffer, the FeIII/II redox wave was replaced by a large irreversible oxygen reduction current, having an Eporr at 250 mV for FeDB and at 260 mV for FeOB. The ORR occurred at a potential similar to the FeIII/II redox potential for FeDB, while for FeOB, the ORR occurred at a cathodic potential relative to the FeIII/II redox potential, implying a redox step in the ORR cycle of FeOB.
Under heterogeneous electrochemical conditions, the rate of O2 binding to ferrous porphyrins was found to be 10^6 M^-1 s^-1 for high-spin FeII species with a rare exception. The reduction of FeIII under a catalytic steady state requires a cathodic ORR potential relative to the FeIII/II potential and a KIE of 10.6.
Outcomes:
- The ORR rates of FeDB and FeOB are 2 to 3 orders of magnitude higher than those of FeTPP under homogeneous conditions in organic solution.
- The rate of O2 binding is enhanced by at least 1 order of magnitude when multiple positively charged residues are introduced in the second sphere of an iron porphyrin.
- Under heterogeneous conditions, the rate of O2 binding to ferrous porphyrins is 10^6 M^-1 s^-1 for high-spin FeII species with a rare exception.
- The experimentally observed ORR rates for these iron porphyrins are orders of magnitude faster than the rates of the ORR based on their overpotentials under both homogeneous and heterogeneous conditions.
Amine Groups in the Second Sphere of Iron Porphyrins Allow for Higher and Selective 4e − /4H + Oxygen Reduction Rates at Lower Overpotentials
Bhunia; Ghatak; Rana; Dey
Full-text link: https://doi.org/10.1021/jacs.2c13552
What this paper is about
- In organic solvents, the rate of the ORR catalyzed by iron porphyrins is the protonation of the Fe III.
- O 2 species is the rds; 4547 however, the situation is quite different under aqueous conditions where the ORR is performed under heterogeneous conditions.
- In situ SERRS-RDE data helps us to identify the species accumulated under the steady state and suggest that the rate enhancement by these iron porphyrins with cationic protonated amine groups in the second sphere is caused by faster O 2 binding and faster OO heterolysis and, more importantly, the rds of the ORR in the aqueous medium changes depending on the nature and number of residues in the second sphere.
What you can learn
- The complexes FeDB and FeOB are drop-casted over an EPG electrode, and then electrochemical data are collected under heterogeneous conditions in pH 7 phosphate buffer solution and compared with those of the previously reported complex.
- In an air-saturated buffer, the Fe III/II redox wave is replaced by a large irreversible oxygen reduction current having an E P ORR at 250 mV for FeDB and at 260 mV for FeOB.
- Note that the O 2 reduction occurs at a potential similar to the Fe III/II redox potential for FeDB, while for FeOB, the ORR occurs at a cathodic potential relative to the Fe III/II redox potential for FeOB, implying that there is a redox step in the ORR cycle of FeOB, which is more uphill than the Fe III/II step, that is, ETPT or PCET.
- Under heterogeneous electrochemical conditions, the rate of O 2 binding to ferrous porphyrins are reported to be 10 6 M 1 s 1 for high-spin Fe II species with a rare exception.
- O 2 under a catalytic steady state requires a cathodic ORR potential relative to the Fe III/II potential and a KIE of 10.6, indicating that the reduction of Fe III.
- For FeDB, the accumulation of the LS Fe III -OOH species during the ORR indicates that the OO bond cleavage is the rds with a rate of 7.26 0.3 10 6 M 1 s 1 which is 125 times faster than the ORR rate of FeEs 4 and is 2 times slower than iron porphyrin with a pendant amine, which has a rate of 13.5 0.9 10 6 M 1 s 1.
- The experimentally observed ORR rates for these iron porphyrins are orders of magnitude faster than the rates of the ORR based on their overpotentials under both homogeneous conditions in organic solution and heterogeneous conditions under aqueous solution.
- Anaerobic measurements were performed inside a glovebox under a N 2 atmosphere, and ORR data were recorded under aerobic conditions in an O 2 saturated acetonitrile medium.
- Zn 2 2H 2 O was added, and the reaction was stirred for 6 h. The solvent was evaporated and extracted with DCM and water.
Q: What is the main focus of the paper?
A: The paper focuses on the synthesis of iron porphyrins and investigates the effect of introducing tertiary amines near the catalytic site on the FeIII/II potential and electrochemical ORR catalysis.
Q: What is the purpose of using in situ SERRS-RDE?
A: In situ SERRS-RDE is used to detect the intermediates formed during the complex reaction landscape of the ORR.
Q: What is the difference in ORR rates between FeDB and FeOB and FeTPP?
A: The ORR rates of FeDB and FeOB are 2 to 3 orders of magnitude higher than those of FeTPP under homogeneous conditions in organic solution.
Q: What is the effect of multiple positively charged residues on the rate of O2 binding?
A: The rate of O2 binding is enhanced by at least 1 order of magnitude when multiple positively charged residues are introduced in the second sphere of an iron porphyryn.
Q: What is the role of amine groups in the second sphere of iron porphyrins?
A: Amine groups in the second sphere of iron porphyrins allow for higher and selective 4e−/4H+ oxygen reduction rates at lower overpotentials.