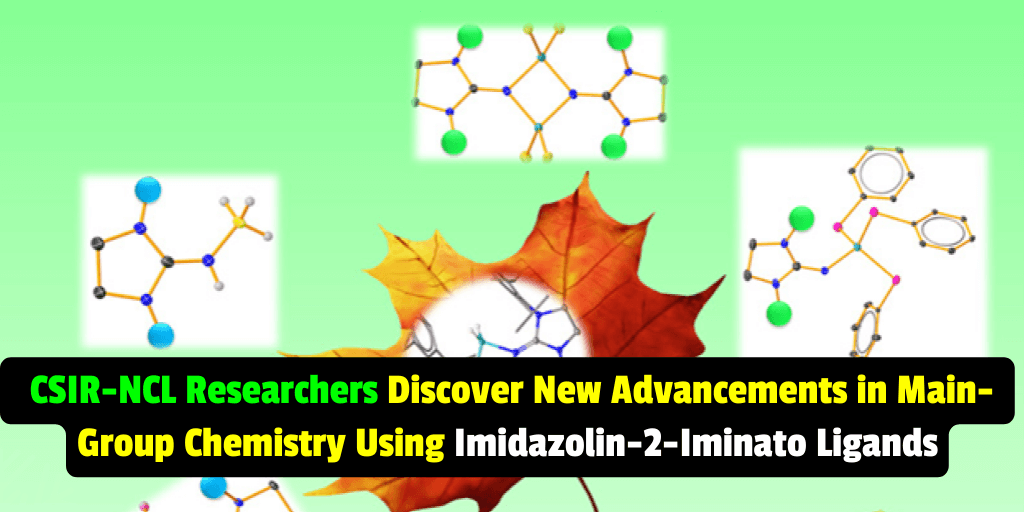
A recent study published in ACS Organometallics has shown the effectiveness of imidazolidin-2-iminato ligands in stabilizing a range of electron-poor main-group species, expanding the use of N-heterocyclic carbenes in main-group chemistry.
Summary:
- Imidazolidin-2-iminato ligands can stabilize a range of electron-poor main-group species.
- NMR spectroscopy, X-ray studies, and mass spectrometric analysis were used to identify the composition and constitution of the compounds
- Imidazolidin-2-iminato ligands have potential applications in organometallic and main-group chemistry.
The study, conducted by researchers Pahar, Kundu, George, Gonnade, and Sen, aimed to investigate the use of imidazolidin-2-iminato ligands in main-group chemistry. N-heterocyclic carbenes, of which imidazolidin-2-iminato ligands are a part, have previously been used in organometallic chemistry but not as extensively in main-group chemistry. However, recent studies by the groups of Rivard and Masuda have shown the potential of imidazolin-2-iminato ligands in stabilizing electron-poor main-group species.
The researchers used proton NMR spectroscopy, single-crystal X-ray studies, and mass spectrometric analysis to identify the composition and constitution of the compounds produced in their reactions. They found that the divergent reactions of BH3SMe2 and BHCl2SMe2 could be attributed to the labile nature of the BCl bond. Additionally, the molecular structure of one compound (8) showed the distorted tetrahedral environment of the aluminum atom, with two nitrogen atoms and two sulfur atoms of the thiol groups.
The study also found that while hydride/methyl exchange took place with BH3SMe, an imidazolidin-2-imine supported BH3 adduct was obtained upon reaction with BH3THF. Furthermore, the synthesis of an analogous bromide derivative was achieved by treating one compound (2) with Br2. The reactions of 2 with diphenyldichalcogenides also resulted in the formation of an imidazolidin-2-iminium cation with a tetra aluminate for sulfur and an imidazolidin-2-imine coordinated triphenylselenyl alane adduct.
Overall, the study highlights the potential of imidazolidin-2-iminato ligands in stabilizing a range of electron-poor main-group species, expanding the use of N-heterocyclic carbenes in main-group chemistry.
Outcomes:
- Imidazolidin-2-iminato ligands have been found to be a formidable class of ligands in organometallic and main-group chemistry, with potential applications in the stabilization of electron-poor main-group species.
- The study used proton NMR spectroscopy, single-crystal X-ray studies, and mass spectrometric analysis to identify the composition and constitution of the compounds produced.
Substitution at a Coordinatively Saturated Aluminum Center Stabilized by Imidazolidin-2-imine Scheme 1. Canonical Forms of Imidazolidin-2-iminato Ligands
Pahar; Kundu; George; Gonnade; Sen
Full text link: https://doi.org/10.1021/acs.organomet.2c00630
About This Research
- N-heterocyclic carbenes are regarded as a formidable class of ligands in organometallic and main-group chemistry.
- 13 It took a while to use these ligands in main-group chemistry, but recently the groups of Rivard, 5 and Masuda have extensively used imidazolin-2-iminato ligands to stabilize a range of remarkable electron-poor main-group species.
- In comparison, the use of related imidazolidin-2-iminato ligands in main-group chemistry is comparatively less, though it has been used in the isolation of a carbene-stabilized phosphorus mononitride.
About Research Work
- In the proton NMR spectrum, there is a broad resonance at 2.41 ppm with the integration of four protons corresponding to the hydrogen atoms of the two AlH 2 moieties.
- The divergent reactions of BH 3 SMe 2 and BHCl 2 SMe 2 can be attributed to the labile nature of the BCl bond, which has also been reported by The composition and constitution of 3 and 4 are elucidated by multinuclear NMR spectroscopy, single-crystal X-ray studies, and mass spectrometric analysis.
- The molecular structure of 8 shows the distorted tetrahedral environment of the aluminum atom with two nitrogen atoms and two sulfur atoms of the thiol groups.
- While the hydride/methyl exchange takes place with BH 3 SMe, an imidazolidin-2-imine supported BH 3 adduct was obtained upon reaction with BH 3 THF.
- The synthesis of an analogous bromide derivative was realized by treating 2 with Br 2.
- The reactions of 2 with diphenyldichalcogenides afforded an imidazolidin-2-iminium cation with a tetra aluminate for sulfur and an imidazolidin-2-imine coordinated triphenylselenyl alane adduct.
Q: What are N-heterocyclic carbenes used for in organometallic and main-group chemistry?
A: N-heterocyclic carbenes are regarded as a formidable class of ligands in organometallic and main-group chemistry. Recently, the groups of Rivard and Masuda have extensively used imidazolin-2-iminato ligands, a type of N-heterocyclic carbenes, to stabilize a range of remarkable electron-poor main-group species.
Q: How is the use of imidazolidin-2-iminato ligands in main-group chemistry compared to the use of imidazolin-2-iminato ligands?
A: In comparison, the use of related imidazolidin-2-iminato ligands in main-group chemistry is comparatively less, though it has been used in the isolation of a carbene-stabilized phosphorus mononitride.
Q: What techniques were used to identify the composition and constitution of the compounds produced in the study?
A: The composition and constitution of the compounds produced in the study were identified using multinuclear NMR spectroscopy, single-crystal X-ray studies, and mass spectrometric analysis.
Q: What is the molecular structure of compound 8?
A: The molecular structure of compound 8 shows the distorted tetrahedral environment of the aluminum atom with two nitrogen atoms and two sulfur atoms of the thiol groups.
Q: What happens when compound 2 is treated with Br2?
A: The synthesis of an analogous bromide derivative was realized by treating compound 2 with Br2.
Q: What are the reactions of compound 2 with diphenyldichalcogenides?
A: The reactions of compound 2 with diphenyldichalcogenides afford an imidazolidin-2-iminium cation with a tetra aluminate for sulfur and an imidazolidin-2-imine coordinated triphenylselenyl alane adduct.