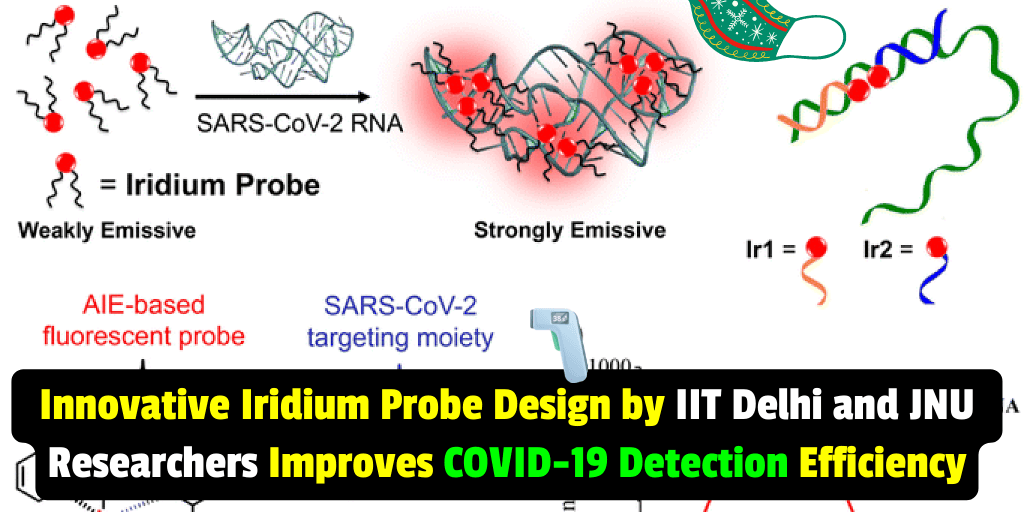
A new study has developed a single-step fluorescence assay that uses iridium-based metal aggregation-induced emission (AIE) luminogens as turn-on fluorescent probes for detecting the RNA of SARS-CoV-2 in 10 minutes at room temperature.
Bullet Point Summary:
- A new study has developed a single-step fluorescence assay for detecting SARS-CoV-2 RNA.
- Iridium-based metal aggregation-induced emission (AIE) luminogens were used as turn-on fluorescent probes.
- The Ir1 probe was more effective than Ir2, with a lower LOD of 1.84 RNA copies.
- The assay has a sensitivity of 90% and a specificity of 80%.
- The detection strategy may be extended to other pathogens in the future.
The COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2 has claimed over 6.5 million human lives so far. In this study, published in Dalton Transactions, researchers have developed a single-step fluorescence assay that uses iridium-based metal aggregation-induced emission (AIE) luminogens (Ir1 and Ir2) as turn-on fluorescent probes for detecting the RNA in 10 min at room temperature. The Ir1 probe performed better than Ir2 and gave a lower LOD of 1.84 RNA copies. The sensitivity and specificity of the probes in clinical samples were found to be 90% and 80%, respectively.
One of the reasons why Ir1 shows better detection efficiency than Ir2 is because the binding energy with the complementary target sequence is higher for Ir1 than for Ir2. Additionally, the fluorescence signal varied with the RNA concentration, which confirms that the viral components induced the AIE phenomenon. This result was also corroborated by dynamic light scattering experiments, which showed that the hydrodynamic diameter of the Ir1 and Ir2 probes increased significantly upon their rapid aggregation in the presence of SARS.
In addition, the researchers also compared their test to other methods reported in the literature and found that their assay performs reasonably well on multiple parameters compared to other COVID-19 detection platforms. The detection strategy may be extended to other harmful pathogens in the future; some work has already been reported.
Outcomes:
- The Ir1 probe performed better than Ir2 and gave a lower detection limit (LOD) of 1.84 RNA copies.
- The sensitivity and specificity of the probes in clinical samples were found to be 90% and 80%, respectively.
- The assay performs reasonably well on multiple parameters compared to other COVID-19 detection platforms reported in the literature.
- The detection strategy may be extended to other harmful pathogens in the future.
Q: What is the study about?
A: The study is about developing a single-step fluorescence assay that uses iridium-based metal aggregation-induced emission (AIE) luminous (Ir1 and Ir2) as turn-on fluorescent probes for detecting the RNA of SARS-CoV-2 in 10 minutes at room temperature.
Q: Which probe performed better in the study?
A: The Ir1 probe performed better than Ir2 and gave a lower detection limit (LOD) of 1.84 RNA copies.
Q: What is the sensitivity and specificity of the assay in clinical samples? A: The assay’s sensitivity in clinical samples is 90%, and the specificity is 80%.
Q: How does the assay compare to other COVID-19 detection platforms reported in the literature?
A: The assay performs reasonably well on multiple parameters compared to other COVID-19 detection platforms reported in the literature.
Q: Can the detection strategy be extended to other harmful pathogens?
A: The detection strategy may be extended to other harmful pathogens in the future; some work has already been reported.
Q: What are the reasons why Ir1 shows better detection efficiency than Ir2?
A: Ir1 shows better detection efficiency than Ir2 because the binding energy with the complementary target sequence is higher for Ir1 than for Ir2. Additionally, the fluorescence signal varied with the RNA concentration, which confirms that the viral components induced the AIE phenomenon. This result was also corroborated by dynamic light scattering experiments, which showed that the hydrodynamic diameter of the Ir1 and Ir2 probes increased significantly upon their rapid aggregation in the presence of SARS.
Q: How are the Iridium probes designed in the study?
A: The Iridium probes are smartly designed such that their distal cyclometalated ends lie near each other when the two probes bind to the same RNA molecule. This means that in addition to the intermolecular probe-probe interactions observed in the single probe experiments, intramolecular probe interactions will also start contributing to the total fluorescence intensity in the mixed probe experiments. This is likely the reason why we see unexpected trends when the concentrations of the Ir1 and Ir2 probes are varied concerning each other.
Dalton Transactions: COVID-19 detection using AIE-active iridium complexes
Li; Dalton; Gupta; Adarsh; Manchanda; Sasmal; Gupta
Full-text link: https://doi.org/10.1039/d2dt03554e
About This Research
- The coV-2 RNA signal was highly specific in our assay.
- For both RNA concentrations, the fluorescence intensity changes were found to be higher in Ir1 compared to those in Ir2, suggesting that Ir1 is a better probe for SARS.
- In addition, the fluorescence signal varied with the RNA concentration, which confirmed that the viral components induced the AIE phenomenon. This result was also corroborated by dynamic light scattering experiments, which showed that the hydrodynamic diameter of the Ir1 and Ir2 probes increased significantly upon their rapid aggregation in the presence of SARS.
Research Work
- One of the reasons why Ir1 shows better detection efficiency than Ir2 is because the binding energy with the complementary target sequence is higher for ASO1 than for ASO2.
- However, since we know that the RNA molecule is monovalent, it is safe to assume that the RNA molecules exist in intermolecular clusters and not as monodispersed entities in solution, which provide multiple adjacent sites for the ASO1/ASO2 probes to bind. This is not entirely unexpected as RNA molecules are known to assemble into elaborate tertiary structures, forming large globular shapes stabilized by various non-sequence-specific molecular interactions.
- The iridium probes are smartly designed such that their distal cyclometalated ends lie near each other when the two probes bind to the same RNA molecule. This means that in addition to the intermolecular probe-probe interactions observed in the single probe experiments, intramolecular probe interactions will also start contributing to the total fluorescence intensity in the mixed probe experiments. This is likely the reason why we see unexpected trends when the concentrations of the Ir1 and Ir2 probes are varied concerning each other.
- CoV-2 N-gene and carry Comparison between our test and other methods reported in the literature.
- The Ir1 probe performed better than Ir2 and gave a lower LOD of 1.84 RNA copies.
- The sensitivity and specificity of the probes in clinical samples were found to be 90% and 80%, respectively.