Researchers have conducted a study on the potential advantages of plasmon-enhanced electrocatalysis (PEEC) for converting electrical energy to chemical energy and vice versa compared to conventional electrocatalysis. Plasmon-enhanced electrocatalysis involves combining localized surface plasmon resonance excitation with electrochemical bias applied to a plasmonic material. The study, titled “Nano-Impact Single-Entity Electrochemistry Enables Plasmon-Enhanced Electrocatalysis,” was published in the journal Angewandte International Edition Chemie.
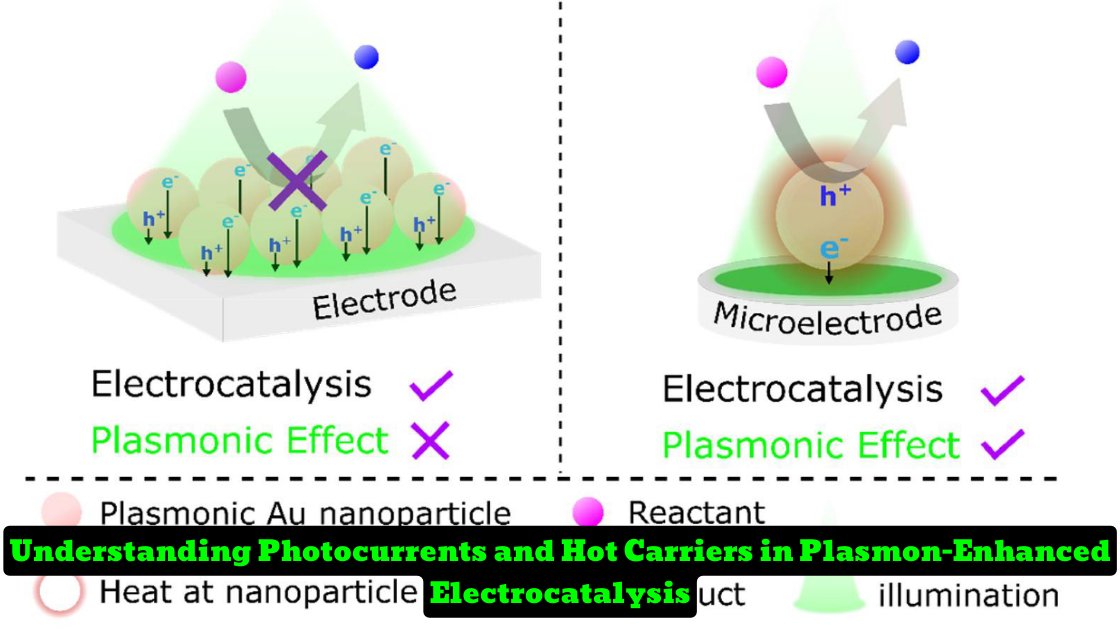
The researchers used nano-impact single-entity electrochemistry (SEE) to investigate the intrinsic activity of plasmonic catalysts at the single-particle level using glucose electrooxidation and oxygen reduction on gold nanoparticles as model reactions. They found that in conventional ensemble measurements, plasmonic effects had minimal impact on photocurrents due to the continuous equilibration of the Fermi level of the deposited gold nanoparticles with the working electrode, leading to fast neutralization of hot carriers by the measuring circuit.
The photocurrents detected in the ensemble measurements were caused by photo-induced heating of the supporting electrode material. In SEE, the EF of suspended gold nanoparticles was unaffected by the working electrode potential, making plasmonic effects the dominant source of photocurrents under SEE experimental conditions.
The study provides insights into the potential advantages and limitations of plasmon-enhanced electrocatalysis and highlights the importance of using techniques such as SEE to investigate the intrinsic activity of plasmonic catalysts at the single-particle level.
Angewandte International Edition Chemie Title: Nano-Impact Single-Entity Electrochemistry Enables Plasmon- Enhanced Electrocatalysis Nano-Impact Single-Entity Electrochemistry Enables Plasmon-Enhanced Electrocatalysis
Ganguli; Zhao; Parlak; Hattori; Sa; Sekretareva; Ganguli; Zhao; Parlak; Hattori; Sá; Sekretareva
Full-text link: https://doi.org/10.1002/anie.202302394
What this paper is about
- Combining LSPR excitation with an electrochemical bias applied to the plasmonic material, known as plasmon-enhanced electrochemistry or plasmon-enhanced electrocatalysis, has the potential to result in more efficient conversion of electrical energy to chemical energy and vice versa compared to conventional electrocatalysis.
- First, LSPR excitation strongly enhances the electric field on the surface of the electrocatalyst.
- In electrocatalytic systems, plasmonic nanomaterials can act as both light-adsorbing and electrocatalytic sites or be combined with other electrocatalytic materials and/or semiconductors Accepted Manuscript.
What you can learn
- They were able to quantify the relative contribution of thermal effects and hot carriers in plasmon-enhanced electrooxidation and assess the thermalized hot carrier energy distribution after the electron injection to the Accepted Manuscript. This study used reactant-free AuNPs with an average size of 18 4 nm as measured by transmission electron microscopy as a plasmonic electrocatalytic material.
- AuNPs in 10 mM NaOH, 50 mM glucose solution experimental conditions in SEE for glucose.1002/anie.202302394.
- Researchers have performed photocurrent measurements of glucose electrooxidation on AuNPs under illumination with a conventional ensemble electrochemistry setup and at the singleparticle level using nano-impact SEE.
- Two irradiation wavelengths, one corresponding to LSPR of AuNPs where generation of hot carriers occurs and one that can not account for any hot charge carrier effects, were chosen to distinguish photothermal from LSPR contribution to electrocatalysis.
- In ensemble measurements on HOPG electrodes with immobilized AuNPs, the wavelength independence of the electrocatalytic photocurrents at 532 nm and 650 nm corresponding to approximately identical absorbance of the electrode suggests a minimal contribution of LSPR to electrocatalysis.
- Temperature effects due to HOPG absorption dominate the observed photocurrent. This is further confirmed by control experiments on AuNP/ITO electrodes, where only a minor current increase of ~4% was detected under 532 nm illumination and 1.7% under 650 nm illumination for glucose electrooxidation.
- Thus, electrocatalysis enhancement by plasmonic effects is minimal for AuNPs immobilized on conductive support in accordance with our earlier results.
- Researchers have shown that the contribution of plasmonic effects to electrocatalysis in conventional ensemble electrochemical systems when there is direct contact between the plasmonic material.1002/anie.202302394.
Core Q&A related to this research
What is plasmon-enhanced electrocatalysis?
Plasmon-enhanced electrocatalysis is a combination of localized surface plasmon resonance excitation and electrochemical bias applied to a plasmonic material that can result in improved electrical-to-chemical energy conversion compared to conventional electrocatalysis.
What is the advantage of using nano-impact single-entity electrochemistry (SEE) in investigating the intrinsic activity of plasmonic catalysts?
The advantage of using nano-impact single-entity electrochemistry (SEE) is that it allows for investigating the intrinsic activity of plasmonic catalysts at the single-particle level using glucose electrooxidation and oxygen reduction on gold nanoparticles as model reactions.
What is the minimal impact of plasmonic effects on photocurrents in conventional ensemble measurements?
In conventional ensemble measurements, the plasmonic effects have minimal impact on photocurrents, which is suggested to be due to the continuous equilibration of the Fermi level of the deposited gold nanoparticles with the Fermi level of the working electrode, leading to fast neutralization of hot carriers by the measuring circuit. The photocurrents detected in the ensemble measurements are caused by photo-induced heating of the supporting electrode material.
What is the dominant source of photocurrents under SEE experimental conditions?
Under SEE experimental conditions, plasmonic effects are the dominant source of photocurrents because the EF of suspended gold nanoparticles is unaffected by the working electrode potential, unlike in conventional ensemble measurements.
Basic Q&A related to this research
What is plasmon-enhanced electrocatalysis?
Plasmon-enhanced electrocatalysis (PEEC) is a process that combines localized surface plasmon resonance (LSPR) excitation with an electrochemical bias applied to a plasmonic material to result in improved electrical-to-chemical energy conversion compared to conventional electrocatalysis.
What is localized surface plasmon resonance (LSPR)?
Localized surface plasmon resonance (LSPR) is a phenomenon that occurs when light interacts with metal nanoparticles, resulting in the excitation of surface plasmons that cause strong enhancement of the electric field on the surface of the electrocatalyst.
What is electrochemical bias?
Electrochemical bias is a voltage applied to a plasmonic material to drive electrochemical reactions and improve the efficiency of electrical-to-chemical energy conversion.
What is electrical-to-chemical energy conversion?
Electrical-to-chemical energy conversion is a process that involves converting electrical energy into chemical energy or vice versa, such as in electrocatalytic reactions.
What is nano-impact single-entity electrochemistry (SEE)?
Nano-impact single-entity electrochemistry (SEE) is a technique that allows for the investigation of the intrinsic activity of plasmonic catalysts at the single-particle level using model reactions such as glucose electrooxidation and oxygen reduction on gold nanoparticles.
What is glucose electrooxidation?
Glucose electrooxidation is a chemical reaction that involves the oxidation of glucose to produce energy or other useful products, such as in biofuel cells.
What is oxygen reduction?
Oxygen reduction is a chemical reaction that involves the reduction of oxygen to produce water or other useful products, such as in fuel cells.
What are gold nanoparticles?
Gold nanoparticles are small particles of gold with sizes in the range of nanometers, commonly used as plasmonic catalysts due to their unique optical and electronic properties.
What are ensemble measurements?
Ensemble measurements refer to measurements of a bulk sample or a large number of particles, as opposed to single-particle measurements.
What is the Fermi level?
The Fermi level is a term used in materials science and solid-state physics to describe the energy level of the highest occupied quantum state at absolute zero temperature.
What are photocurrents?
Photocurrents are electric currents generated by the absorption of light in a material.
What are hot carriers?
Hot carriers are energetic electrons or holes that are generated when light is absorbed by a material, which can contribute to the efficiency of plasmon-enhanced electrocatalysis.