Summary:
Researchers have investigated the effects of co-doping metalloids (Ga3+, In3+, Al3+) and chromium (Cr3+) in graphene oxide (GO) adorned zinc sulfide (ZnS) nanowires to enhance their photocatalytic properties. The co-doping resulted in the formation of more defects, leading to higher efficiency in dye degradation. The crystallographic study confirmed the wurtzite crystal structure of the nanowires, with variations observed after co-substitution. The nanowires exhibited improved properties compared to single metal-doped ZnS nanomaterials.
Facts:
- 🧪 Metalloids (Ga3+, In3+, Al3+) and chromium (Cr3+) were co-doped into graphene oxide (GO) adorned zinc sulfide (ZnS) nanowires.
- 🌐 The co-doped nanowires showed enhanced photocatalytic properties and higher efficiency in dye degradation.
- 📐 Crystallographic analysis confirmed the wurtzite crystal structure of the nanowires.
- 🔄 Co-substitution of metalloids and chromium resulted in the formation of more defects, leading to improved properties.
- 🔬 The nanowires exhibited better results compared to single metal-doped ZnS nanomaterials.
- 📊 The crystallite size, lattice parameters, dislocation density, microstrain, stacking faults, and volume were analyzed.
- 🎯 The study highlights the potential of co-doping strategies to enhance the photocatalytic activity of nanomaterials.
In this study, researchers investigated the effects of co-doping metalloids (Ga3+, In3+, Al3+) and chromium (Cr3+) in graphene oxide (GO) adorned zinc sulfide (ZnS) nanowires to improve their photocatalytic properties. The co-doping process resulted in the formation of more defects in the nanowires, which played a crucial role in enhancing the efficiency of dye degradation during photocatalysis.
The crystallographic study revealed that the nanowires exhibited a wurtzite crystal structure, as confirmed by X-ray diffraction (XRD) analysis. The XRD peaks matched well with the JCPDS reference pattern. However, after co-substitution, variations in crystal structure and crystallinity were observed. The crystallite size was calculated using the Debye Scherrer formula, showing an increase in size after co-doping.
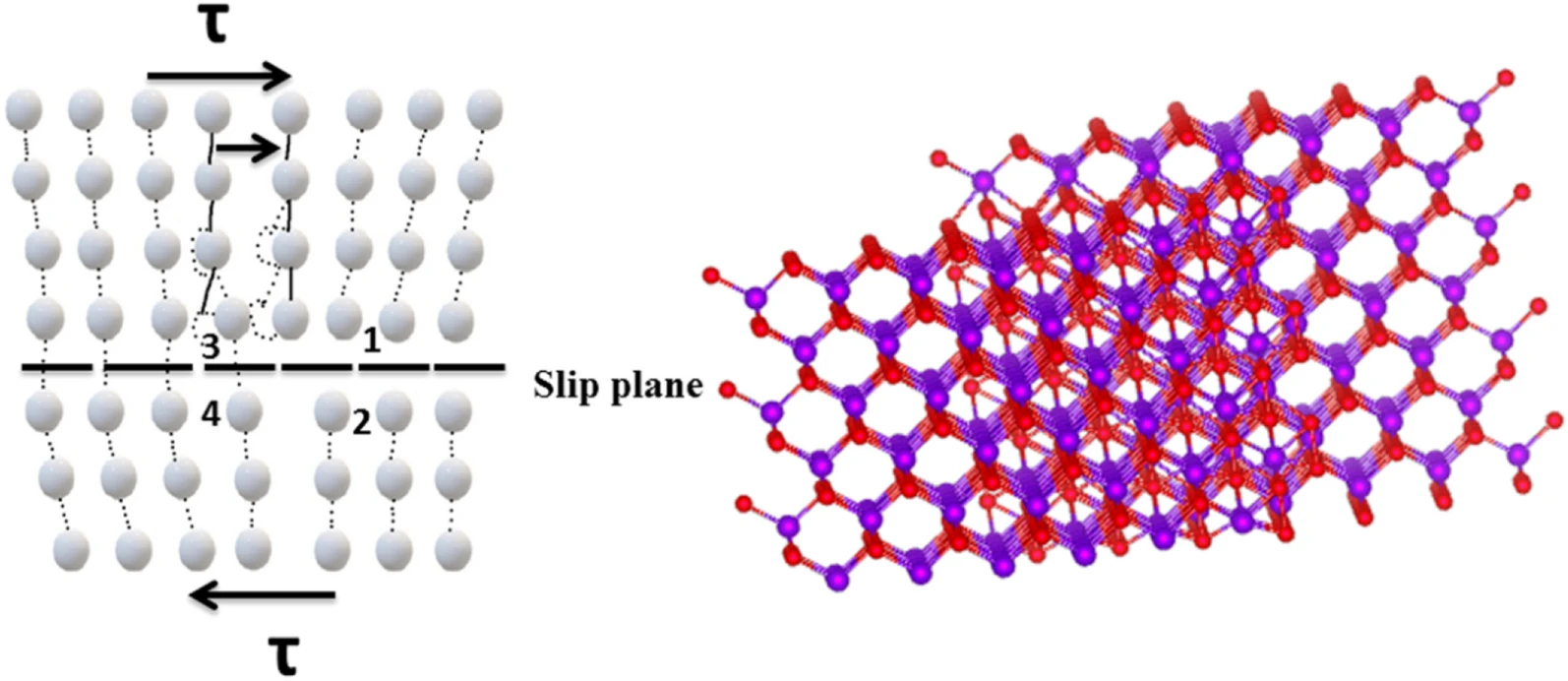
The lattice parameters, including ‘a’ and ‘c’, were determined using the XRD data. The co-substituted nanowires exhibited significant changes in crystal structure compared to the pristine ZnS nanowires. Dislocation density, microstrain, and stacking faults were also analyzed, indicating the presence of extended defects caused by lattice mismatch.
The co-doped ZnS nanowires showed better results in terms of photocatalytic activity compared to single metal-doped ZnS nanomaterials. The introduction of metalloids and chromium into the ZnS lattice enhanced the photocatalytic efficiency, making the nanowires more effective in dye degradation. The study highlights the potential of co-doping strategies to improve the properties of nanomaterials for various applications, including photocatalysis.
Q & A related to this research
Q: What are metalloids?
A: Metalloids are elements that have properties intermediate between metals and nonmetals. They exhibit characteristics of both metal and nonmetal elements and are found in the periodic table along the “staircase” line. Some examples of metalloids include silicon, germanium, and arsenic.
Q: What is co-doping?
A: Co-doping refers to the process of introducing two or more different dopant elements into a material simultaneously. Dopants are impurities deliberately added to alter the properties of a material. Co-doping can be used to modify the electrical, optical, or magnetic properties of a material and is often done to enhance its performance or tailor its characteristics for specific applications.
Q: What is graphene oxide?
A: Graphene oxide is a derivative of graphene, a two-dimensional carbon material consisting of a single layer of carbon atoms arranged in a hexagonal lattice. Graphene oxide is produced by oxidizing graphene, resulting in the introduction of oxygen-containing functional groups. It has unique properties and is used in various fields, including electronics, energy storage, and biomedical applications.
Q: What is zinc sulfide?
A: Zinc sulfide is a compound made up of zinc and sulfur atoms. It is a versatile material with wide-ranging applications, including as a phosphor in fluorescent lights, a component in pigments, a transparent conductor in electronics, and a semiconductor in optoelectronic devices.
Q: What are nanowires?
A: Nanowires are extremely thin structures with diameters on the nanometer scale (typically less than 100 nanometers). They can be composed of various materials, such as metals, semiconductors, or oxides. Nanowires possess unique electrical, optical, and mechanical properties, and they find applications in nanotechnology, electronics, sensors, and energy devices.
Q: What is photocatalytic activity?
A: Photocatalytic activity refers to the ability of a material to accelerate or promote a chemical reaction when exposed to light. In photocatalysis, the material, known as a photocatalyst, absorbs photons from light and utilizes the energy to drive chemical reactions, often involving the transfer of electrons or generation of reactive oxygen species. Photocatalytic activity has applications in areas such as environmental remediation, water purification, and solar energy conversion.
Q: What is a crystallographic study?
A: A crystallographic study involves the analysis and characterization of the crystal structure of a material. It employs techniques such as X-ray crystallography or electron diffraction to determine the arrangement and positions of atoms within a crystal lattice. Crystallographic studies provide insights into the physical and chemical properties of materials and are essential for understanding their behavior and functionality.
Q: What are defects in materials?
A: Defects in materials refer to irregularities, impurities, or structural deviations from the ideal lattice arrangement in a crystalline material. These defects can include missing atoms (vacancies), extra atoms (interstitials), dislocations, grain boundaries, or dopant atoms. Defects can significantly influence the properties and behavior of materials, such as their mechanical strength, electrical conductivity, or optical properties.
Q: What is dye degradation?
A: Dye degradation refers to the process of breaking down or decomposing organic dyes into simpler molecules or compounds.
Research Paper



[…] Co-doping of Metalloids and Chromium in Graphene Oxide Decorated ZnS Nanowires… […]