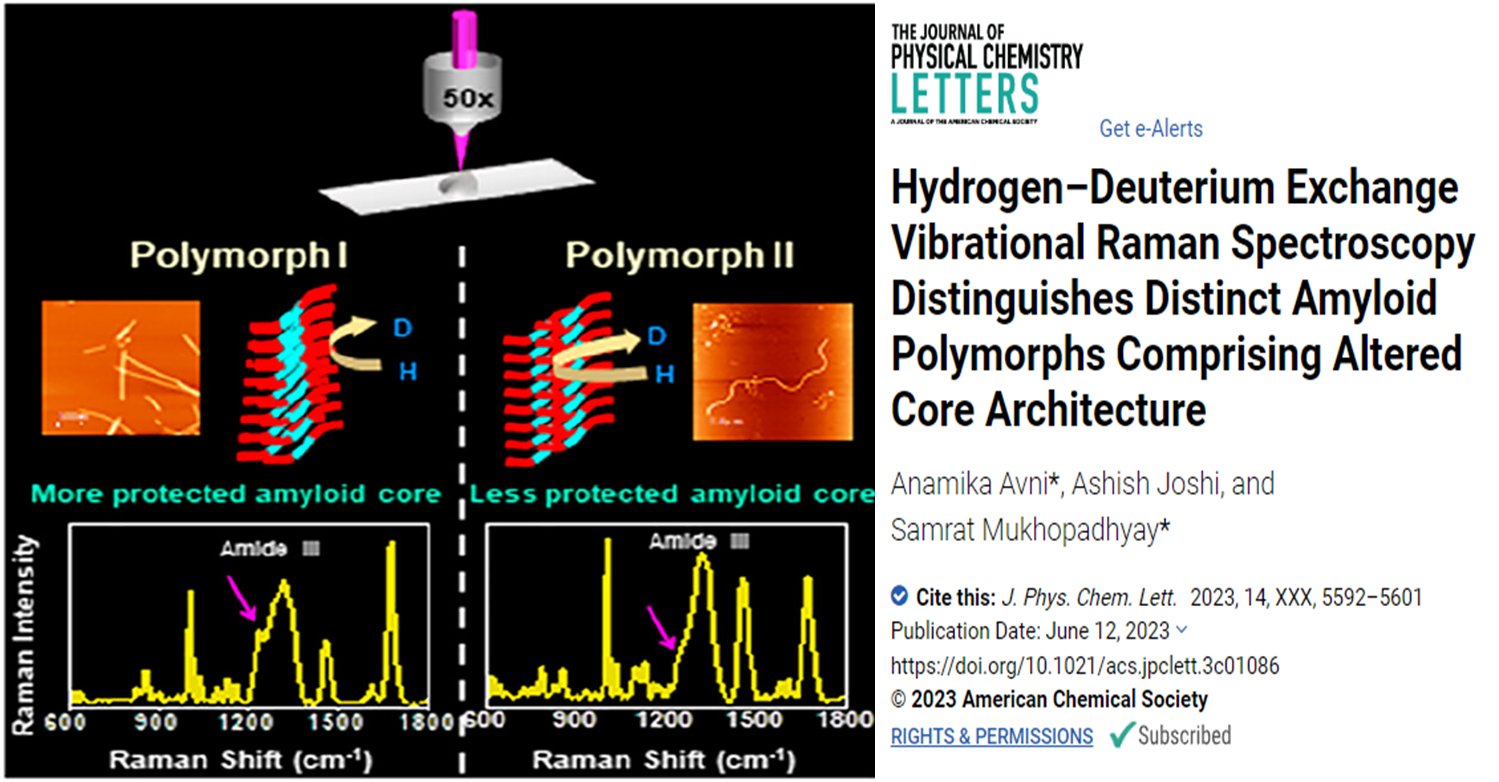
Amyloid fibrils are protein aggregates implicated in various neurological disorders. Recent studies have shown that these fibrils exhibit structural polymorphism, which can contribute to disease phenotypes. In this research, vibrational Raman spectroscopy coupled with hydrogen/deuterium exchange was used to study the structural heterogeneity of different amyloid fibril polymorphs generated in vitro. The spectroscopic analysis revealed distinct conformational signatures and secondary structural compositions for each polymorph.
Facts
- 👥 Amyloid fibrils are protein aggregates implicated in neurological disorders.
- 🧪 Recent studies have revealed that amyloid fibrils exhibit molecular-level polymorphism.
- 🧠 α-synuclein (α-syn) is an intrinsically disordered protein that self-assembles into different fibrillar assemblies and exhibits prion-like strain phenomena.
- 🧬 Environmental factors and post-translational modifications can influence the polymorphic landscape of amyloid assembly.
- 📊 Various structural methods, such as solid-state NMR and cryo-electron microscopy, have been used to determine the atomic structures of amyloid fibrils.
- 💡 Obtaining high-resolution atomic structures of amyloid fibrils can be challenging and requires specialized techniques and equipment.
- 🔬 Vibrational Raman spectroscopy coupled with hydrogen/deuterium exchange was used to characterize the structural heterogeneity of α-synuclein fibril polymorphs.
Amyloid fibrils are protein aggregates that have been linked to various neurological disorders. Recent studies have shown that these fibrils can exhibit structural polymorphism, meaning they can adopt different conformations and exhibit distinct molecular properties. This structural diversity is thought to be responsible for the different disease phenotypes observed.
One particular protein called α-synuclein (α-syn) is an intrinsically disordered protein that self-assembles into fibrillar assemblies. These assemblies can exhibit prion-like strain phenomena, where they can propagate their conformational states and induce the formation of similar fibrils in other cells. The accumulation of α-syn fibrils in nerve and glial cells leads to the development of pathological lesions called Lewy bodies and Lewy neurites, which are characteristic of several neurodegenerative disorders including Parkinson’s disease.
Various factors such as post-translational modifications, primary amino acid sequence variations, temperature, pH, and ionic strength, significantly affect the polymorphic nature of amyloid assembly. These factors can alter the interactions between the protein chains and affect the conformation and pathology of the fibrillar assemblies.
To determine the atomic structures of amyloid fibrils, various structural methods have been employed, including solid-state NMR, cryo-electron microscopy, and X-ray analysis. These techniques provide detailed information about the fibril architecture, packing arrangement, and atomic-level interactions. However, these methods often require specialized labeling or extensive sample preparation, and they can be costly and technically challenging.
In this study, the researchers used vibrational Raman spectroscopy coupled with hydrogen/deuterium exchange to investigate the structural characteristics of amyloid fibril polymorphs generated in vitro. Raman spectroscopy provides information about the vibrational modes of the molecules, which can be used to analyze secondary structural elements and conformational variations. By incorporating hydrogen/deuterium exchange, which is sensitive to the hydrogen bonding patterns within the fibrils, the researchers could probe the supramolecular packing and hydrogen bonding strength of different polymorphs.
However, obtaining high-resolution atomic structures of amyloid fibrils can be challenging and often requires specialized sample preparation and expensive instrumentation. The results of the study demonstrated that vibrational Raman spectroscopy coupled with hydrogen/deuterium exchange could distinguish distinct conformations and secondary structural compositions.
- Anamika Avni*, Ashish Joshi, and Samrat Mukhopadhyay*
- Indian scientist Samrat Mukhopadhyay works at the Indian Institute of Science Education and Research in Mohali, Punjab, focusing on protein science, design and engineering in the Department of Chemical Sciences and Department of Biological Sciences.
- J. Phys. Chem. Lett. 2023
- Publication Date:June 12, 2023
- SUBJECTS:Amides, Chemical structure, Nanofibers, Peptides and proteins, Raman spectroscopy