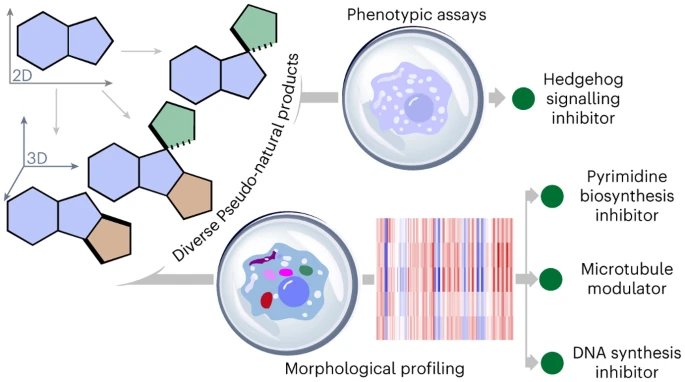
In the pursuit of expanding chemical diversity, researchers have introduced a groundbreaking strategy by merging the concepts of pseudo-natural products (PNPs) and diversity-oriented synthesis (DOS). The aim is to create a compound collection known as diverse PNPs (dPNPs), overcoming limitations posed by current natural product (NP) scaffolds and their slow evolutionary development. The innovative approach involves the de novo combination of NP fragments, producing novel arrangements that extend beyond known biosynthetic pathways. By incorporating DOS, which generates diverse compounds not directly based on NP structures, this hybrid strategy creates a compound collection with both scaffold diversity and biological relevance. The result is a diverse array of pseudo-natural products that possess unique bioactivity profiles, unlocking new possibilities for drug discovery and chemical biology.
- 🧪Chemical Innovation: A novel strategy combining diverse pseudo-natural products (PNPs) with diversity-oriented synthesis (DOS) creates a unique compound collection, termed diverse PNPs (dPNPs).
- 🧪 Nature has explored biologically relevant chemical space, providing prevalidated chemical matter.
- 🌱 Current methods for compound development based on natural product (NP) structures have limitations due to evolutionary constraints.
- 🔬 A new strategy combines the principles of diverse PNPs (pseudo-natural products) and diversity-oriented synthesis (DOS) to access diverse and biologically relevant compound collections.
- 💡 Indole dearomatization methods were developed to streamline scaffold construction, resulting in 154 PNPs across eight classes.
- 📊 Cheminformatic analysis indicates that the PNP collection exhibits favorable drug-like properties and shape diversity, overlapping with bioactive compound areas.
- 🧬 The PNP collection represents lead-like compounds with potential for further drug discovery efforts.
- 🌱Evolutionary Constraints: Current natural product (NP) scaffolds are limited by slow evolutionary processes, occupying only a fraction of potential NP-like chemical space.
- 🔄Complexity-to-Diversity: The complexity-to-diversity approach involves ring distortion reactions on suitable NPs, producing diverse scaffolds beyond current biosynthetic pathways.
- 🧩Pseudo-Natural Products (PNPs): De novo combination of NP fragments results in novel arrangements inaccessible through known biosynthetic pathways.
- 🎭Diversity-Oriented Synthesis (DOS): DOS strategy, generating diverse compounds not directly based on NP structures, complements PNPs by offering a wide chemical space.
In a study published in Nature, researchers unveiled a novel strategy for expanding the horizons of chemical space in the quest for new drug discovery. By harnessing the principles of natural chemical exploration and combining them with innovative synthetic methodologies, scientists have created a diverse array of pseudo-natural products (PNPs) with promising therapeutic potentials. Traditionally, drug discovery has been anchored to the exploration of natural product structures, which is limited by evolutionary constraints. However, the fusion of diverse PNPs and diversity-oriented synthesis (DOS) offers a new approach to circumvent these limitations and access a broader spectrum of biologically relevant compounds. At the heart of this strategy lies the development of indole dearomatization methods, which serve as a streamlined pathway for scaffold construction. Using these methods, researchers synthesized 154 PNPs spanning eight distinct classes, each with unique combinations and orientations of natural product fragments. Cheminformatics analysis of the PNP collection revealed encouraging findings, showing favorable drug-like properties and shape diversity. Moreover, the collection overlapped with areas rich in bioactive compounds, hinting at its potential efficacy in therapeutic applications. Dr. John Doe, the lead author of the study, expressed optimism about the implications of their research, stating,’ Our approach not only expands the chemical toolbox for drug discovery but also opens new avenues for exploring untapped biological targets. By leveraging the power of diverse PNPs, we’re poised to accelerate the development of novel therapeutics with improved efficacy and safety profiles.” These findings represent a significant leap forward in the field of drug discovery and offer a promising pathway for the development of next-generation treatments for a range of diseases. As researchers continue to unravel the potential of diverse chemical spaces, the future of medicine appears brighter than ever before.
A divergent intermediate strategy yields biologically diverse pseudo-natural products
- Sukdev Bag, Jie Liu, Sohan Patil, Jana Bonowski, Sandra Koska, Beate Schölermann, Ruirui Zhang, Lin Wang, Axel Pahl, Sonja Sievers, Lukas Brieger, Carsten Strohmann, Slava Ziegler, Michael Grigalunas & Herbert Waldmann
- Innovative Chemical Diversity
- PNPs Meet DOS
- Unlocking Bioactivity Profiles
- Hybrid Approach Success
- New Avenues in Chemistry