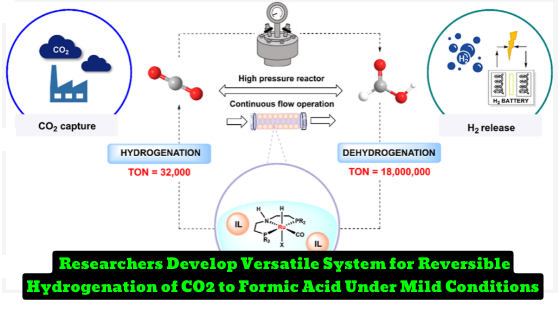
Researchers Develop Versatile System for Reversible Hydrogenation of CO2 to Formic Acid Under Mild Conditions. Versatile CO2 Hydrogenation−Dehydrogenation Catalysis with a Ru−PNP/Ionic Liquid System” that introduces a new catalytic system for the reversible hydrogenation of CO2 to formic acid (FA) under very mild conditions. The system shows extreme flexibility, stability, and reversibility under a wide range of temperatures, pressures, operating times, and catalyst loadings, making it a promising candidate for energy storage technologies based on H2–FA
Summary:
- Researchers have developed a catalytic system for the reversible hydrogenation of CO2 to formic acid (FA) under very mild conditions.
- The system consists of a base-activated pincer and a Ru(H)2(CO)(iPrPNP) pincer, which facilitates both hydrogenation and dehydrogenation of FA.
- The system demonstrated high activity and stability in CO2 hydrogenation already under ambient conditions, leading to STYs of FA up to 0.33 mol L–1 h–1 at 25°C and 1.03 mol L–1 h–1 at 80°C.
- The catalytic system is a promising candidate for energy storage technologies based on H2-FA, presenting practical advantages such as storage and easy manipulations under ambient conditions.
A new homogeneous catalytic approach for the hydrogenation of CO2 to formic acid (FA) and the reverse FA dehydrogenation has been developed by researchers. The catalytic system consists of a base-activated pincer (PNP = bis alkyl- or aryl ethylphosphinoamine) and a Ru(H)2(CO)(iPrPNP) pincer, which is highly active in facilitating both hydrogenation and dehydrogenation of FA. The system has been tested under various conditions, including 25°C for 18 hours under 10:20 bar pressure of CO2/H2, 80°C for 112 days under ambient conditions, and 95°C under octaneous conditions with a catalyst loading of 0.025 mmol of Ru-7 in BMIM OAc (4 mL) to achieve a volume ratio between consumed FA and reactor size of >3,600. The system remained active for dehydrogenation of a continuous flow of FA for up to 4 months, achieving an overall TON exceeding 18 million corresponding to a STY of CO2 and H2 of almost 35.7 mol L–1 h–1, the best result to date in terms of both catalyst activity and stability. The introduced catalytic system is versatile, stable, and reversible under a wide range of temperatures, pressures, operating times, and catalyst loadings, making it a promising candidate for energy storage technologies based on H2-FA presenting practical advantages such as storage and easy manipulations under ambient conditions.
Outcomes:
- The development of a catalytic system for reversible hydrogenation of CO2 to formic acid under mild conditions
- High activity and stability of the catalytic system in CO2 hydrogenation already under ambient conditions
- Promising candidate for energy storage technologies based on H2-FA, presenting practical advantages such as storage and easy manipulations under ambient conditions
Versatile CO 2 Hydrogenation−Dehydrogenation Catalysis with a Ru−PNP/Ionic Liquid System
Piccirilli; Rabell; Padilla; Riisager; Das; Nielsen
Full text link: https://doi.org/10.1021/jacs.2c10399
What this paper is about
- Pincer complexes have been used for a plethora of hydrogenation and dehydrogenation reactions, including the hydrogenation of CO 2 to FA as well as the dehydrogenation of FA for H 2 production.
- H 2 gas mixture or a two-step approach where first the CO 2 was captured by the IL followed by subsequent addition of H 2 for the hydrogenation process promoted by the RuPNP catalyst.
- After completion of the reaction, IL-trapped CO 2 and the Ru-3 complex were observed when using Ru-1 as the catalyst.
What you can learn
- However, a significant amount of CO was detected under such an operation. This work demonstrates a new homogeneous catalytic approach for the hydrogenation of CO 2 to FA and the reverse FA dehydrogenation based on RuPNP complexes in IL.
- The catalytic system showed high activity and stability in CO 2 hydrogenation already under ambient conditions, leading to STYs of FA up to 0.33 mol L 1 h 1 at 25C and 1.03 mol L 1 h 1 at 80C.
- All these features render the system a promising candidate for energy storage technologies based on H 2 FA presenting practical advantages such as storage and easy manipulations under ambient conditions, as well as the possibility to dehydrogenate neat FA maximizing the atom efficiency and hydrogen gravimetric content of the system.
Core Q&A related to this research
- What is the purpose of the catalytic system developed in this study?
- The purpose of the catalytic system is to facilitate the reversible hydrogenation of CO2 to formic acid (FA) under very mild conditions.
- What are the catalysts used in the catalytic system?
- The catalysts used in the catalytic system are composed of a base-activated pincer (PNP = bis alkyl- or aryl ethylphosphinoamine) and a Ru(H)2(CO)(iPrPNP) pincer.
- What are the conditions under which the catalytic system was tested?
- The system was tested at 25°C for 18 h under 10:20 bar pressure of CO2/H2 and at 80°C for 112 days under ambient conditions.
- What is the total turnover number (TON) achieved in the final experiment?
- The TON achieved in the final experiment was 18,100,000.
- What are the advantages of the catalytic system for energy storage technologies?
- The system is versatile, stable, and reversible under a wide range of temperatures, pressures, operating times, and catalyst loadings. It presents practical advantages such as storage and easy manipulations under ambient conditions, as well as the possibility to dehydrogenate neat FA maximizing the atom efficiency and hydrogen gravimetric content of the system.
Basics Q&A related to this research
- What is CO2 hydrogenation?
CO2 hydrogenation is a chemical reaction that converts carbon dioxide (CO2) into other valuable chemicals, such as formic acid (FA) or methanol, by adding hydrogen (H2) gas.
- What is formic acid?
Formic acid (FA) is a colorless liquid with a pungent odor that can be used as a preservative, disinfectant, or reducing agent in various industries.
- What is reversible hydrogenation?
Reversible hydrogenation is a process in which hydrogen (H2) can be added or removed from a molecule, depending on the reaction conditions. In the context of CO2 hydrogenation, reversible hydrogenation refers to the conversion of CO2 into FA and back into CO2 using a catalyst.
- What is a catalytic system?
A catalytic system is a set of chemical reactions or processes that require a catalyst to proceed at a reasonable rate. In CO2 hydrogenation, a catalyst is needed to speed up the reaction and increase its efficiency.
- What are Ru-PNP complexes?
Ru-PNP complexes are ruthenium-based catalysts that are used for CO2 hydrogenation and formic acid dehydrogenation. They consist of a base-activated pincer and a ruthenium metal center that are highly active in facilitating both hydrogenation and dehydrogenation of FA.
- What is an ionic liquid system?
An ionic liquid system is a type of solvent that is composed entirely of ions and has several unique properties, such as high thermal stability, low volatility, and high ionic conductivity. In the context of CO2 hydrogenation, an ionic liquid system can be used to dissolve and transport the reactants and catalysts.
- What are energy storage technologies?
Energy storage technologies are methods of storing energy for later use, such as batteries, fuel cells, or capacitors. In the context of CO2 hydrogenation, energy storage technologies can be based on formic acid dehydrogenation, which releases hydrogen gas as a clean and sustainable fuel.
- What is hydrogen storage?
Hydrogen storage refers to the process of storing hydrogen gas for later use as a fuel source. In the context of CO2 hydrogenation, formic acid can be used as a hydrogen storage medium, as it can be easily dehydrogenated to release hydrogen gas.
- What are PEM fuel cell applications?
PEM fuel cell applications refer to the use of proton exchange membrane (PEM) fuel cells for power generation in various applications, such as electric vehicles, portable devices, or stationary power plants. In the context of CO2 hydrogenation, formic acid dehydrogenation can provide a clean and efficient source of hydrogen gas for PEM fuel cells.
- What is atom efficiency and hydrogen gravimetric content?
Atom efficiency refers to the percentage of atoms in the reactants that are incorporated into the desired product, without generating any waste or byproducts. Hydrogen gravimetric content refers to the amount of hydrogen gas that can be released per unit weight of the formic acid storage medium. In the context of CO2 hydrogenation, maximizing the atom efficiency and hydrogen gravimetric content can improve the efficiency and sustainability of the overall system.