India Researchers at the ITCP – Institute for chemical technology and polymer chemistry (ITCP) have made a discovery in the field of catalytic reactions. The team, consisting of experts in various scientific disciplines, has successfully synthesized atomically dispersed late transition metals supported over ceria.
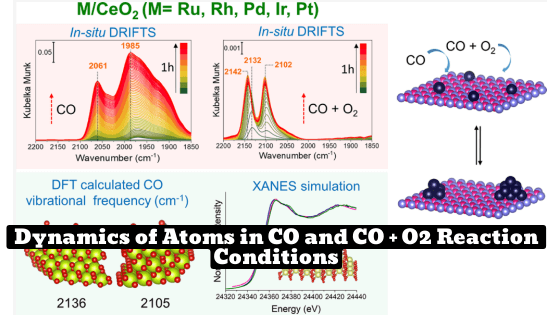
Utilizing advanced spectroscopic techniques such as X-ray absorption spectroscopy and diffuse reflectance infrared Fourier transform, the researchers were able to gain deep insight into the dynamics of atoms during the CO oxidation reaction. The study, which serves as an important benchmark for future research, was carried out under varying reaction conditions and helped to establish a correlation between vibrational shifts and the charge state of single-atom catalysts. The findings by the India Researchers at ITCP, Germany are a significant step forward in understanding the complex interactions that occur during catalytic reactions.
Bullet Point Summary:
- Atomically dispersed late transition metals synthesized with precipitation and wet impregnation methods followed by calcination.
- In-situ DRIFTS and XAS spectroscopy used to investigate local structure and dynamic changes.
- CO vibrational frequencies shifted to higher wavenumbers when gas mixture changed from CO to CO + O2.
- DFT calculations helped identify single sites and preferred locations of SACs with different formal charges.
- Ru and Rh single atoms showed higher stability over CeO.
India Researchers at ITCP, Germany, has conducted a study on the dynamics of atomically dispersed late transition metals (Ru, Rh, Pd, Ir, and Pt) supported over ceria for CO reaction. The study used various spectroscopic techniques, including in-situ X-ray absorption spectroscopy (XAS) and diffuse reflectance infrared Fourier transform (DRIFTS) to understand the dynamics of atoms under CO and CO + O2 reaction conditions. The results showed that when the gas mixture was changed from CO to CO + O2, all SACs were in the oxidized form and the CO vibrational frequencies were shifted to higher wavenumbers due to the strong binding of CO to the single atom.
Additionally, the study used density functional theory (DFT) calculations to identify the CO vibrational frequencies and correlate these values to the corresponding Bader charge and CO binding energies of the atomically dispersed catalysts (SACs). The calculated phase diagram showed the preferred locations of the SACs with different formal charges over CeO and surfaces in a temperature range up to 800 K. The results showed that Ru and Rh single atoms showed higher stability over CeO.
The study serves as an important benchmark in identifying and characterizing the dynamic structure of atomically dispersed catalysts for CO reaction, and the correlation of vibrational shifts with the charge state of SAC will give important insights into the local surface structure, thus helping to establish structure-activity relationships in future studies.
Outcomes:
- India Researchers at ITCP, Germany, have synthesized atomically dispersed late transition metals (Ru, Rh, Pd, Ir, and Pt) supported over ceria.
- In-situ X-ray absorption spectroscopy (XAS) and diffuse reflectance infrared Fourier transform (DRIFTS) were used to understand the dynamics of atoms under CO and CO + O2 reaction conditions.
- Density functional theory (DFT) calculations were carried out to identify the CO vibrational frequencies, and the values were correlated to the corresponding Bader charge and CO binding energies of the atomically dispersed catalysts (SACs).
Tracking and Understanding Dynamics of Atoms and Clusters of Late Transition Metals with In-Situ DRIFT and XAS Spectroscopy Assisted by DFT
Sarma; Jelic; Neukum; Doronkin; Huang; Studt; Grunwaldt
Full text link: https://doi.org/10.1021/acs.jpcc.2c07263
What this paper is about
- For the CO oxidation reaction that was reported to be catalyzed by a Pt single site over CeO, 21 recent operando investigations have shown that Pt clusters are true active species and the single sites act as spectators.
- In this work, we have synthesized atomically dispersed late transition metals supported over ceria with precipitation and wet impregnation methods followed by calcination at 623 K and 1073 K in static air, respectively.
- Various spectroscopic techniques were used to investigate the local structure of the synthesized catalysts, and in-situ diffuse reflectance infrared Fourier transform spectroscopy and XAS were used to understand the dynamic structural changes.
What you can learn
- To investigate the stability of two series of differently synthesized SACs containing noble metals at room temperature, we performed a series of in-situ DRIFTS and XAS experiments under reducing and oxidizing atmospheres.
- Both DRIFTS and XAS are complementary to each other, and we noticed that in the case of 5d metals, changes in the standard XANES regions were not so obvious, while DRIFTS showed clear changes in CO vibrational frequencies.
- The reason for this is that XAS is a bulk technique and small changes might get averaged out, in particular when the single-atom sites are very heterogeneous in nature.
- When the gas mixture was changed from CO to CO + O, all SACs were in the oxidized form and the CO vibrational frequencies were shifted to higher wavenumbers, as CO was strongly bound to the single atom due to the lack of backdonation from the metal to CO; less availability of electrons in the antibonding orbital of CO was observed. This trend is observed for all SACs, irrespective of the method of synthesis. This highlights the dynamic nature of SACs and shows how different species can evolve over time depending on reaction conditions.
- CO insertion is a highly important reaction and can only be investigated in detail through SACs, as they closely resemble molecular catalysts.
- DFT calculations helped to identify single sites, and the calculated phase diagram showed the preferred locations of the SACs with different formal charges over CeO and surfaces in a temperature range up to 800 K. Ru and Rh single atoms showed higher stability over CeO, as we observed in the temperature-programmed reduction XANES experiments.
Q: What is this paper about?
A: This paper is about synthesizing atomically dispersed late transition metals supported over ceria and investigating the local structure and dynamic structural changes of the synthesized catalysts through in-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and X-ray absorption spectroscopy (XAS), assisted by density functional theory (DFT) calculations.
Q: What methods were used to synthesize the catalysts?
A: The catalysts were synthesized using precipitation and wet impregnation methods followed by calcination at 623 K and 1073 K in static air.
Q: What spectroscopic techniques were used to investigate the local structure of the catalysts?
A: The local structure of the catalysts was investigated using in-situ DRIFTS and XAS spectroscopy.
Q: What did the in-situ DRIFTS and XAS experiments reveal about the dynamic structural changes of the catalysts?
A: The in-situ DRIFTS and XAS experiments showed the dynamic nature of single-atom catalysts (SACs) and how different species can evolve over time depending on reaction conditions. The CO vibrational frequencies shifted to higher wavenumbers when the gas mixture was changed from CO to CO + O2, and this trend was observed for all SACs.
Q: How did DFT calculations help in this study?
A: DFT calculations helped to identify the single sites and showed the preferred locations of the SACs with different formal charges over CeO and surfaces in a temperature range up to 800 K.