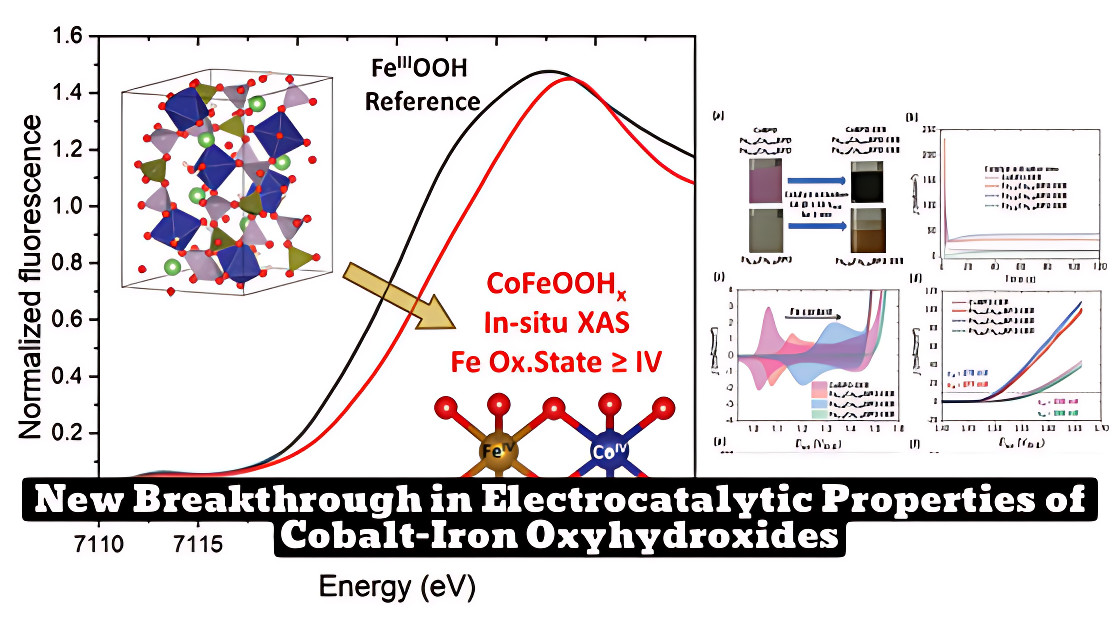
Scientists have synthesized Fe1-xCoxBPO (cobalt-iron oxyhydroxides after reconstruction) samples and improved their electrocatalytic properties by reducing the particle size. Quasi in situ X-ray absorption spectroscopy (XAS) was used to investigate the electronic and structural behavior of the active phase and revealed redox-active Fe with oxidation states higher than three.
In situ EXAFS data showed a substantial cobalt reoxidation behavior and a shorter Fe-O bond length. The quasi in situ XAS measurements of the samples demonstrated strong interactions between iron and cobalt, leading to a redox activity of the iron centers with oxidation states up to 3.2.
Bullet Point Summary:
- Fe1-xCoxBPO samples were synthesized with a mild hydrothermal approach and reduced particle size through high-energy ball-milling.
- Quasi in situ XAS was used to investigate the electronic and structural behavior of the active phase, revealing redox-active Fe with oxidation states >3.
- In situ EXAFS data showed substantial cobalt reoxidation behavior and a shorter Fe-O bond length.
- Quasi in situ XAS measurements demonstrated strong interactions between iron and cobalt, leading to a redox activity of the iron centers with oxidation states up to 3.2.
The study, “In Situ Detection of Iron in Oxidation States ≥ IV in Cobalt-Iron Oxyhydroxide Reconstructed during Oxygen Evolution Reaction” was conducted by researchers Reith, Hausmann, Mebs, Mondal, Dau, Driess, and Menezes. The Fe1-xCoxBPO samples were synthesized using a mild hydrothermal approach and reduced in particle size through high-energy ball-milling. The electrocatalytic properties of the samples were then studied using quasi in situ X-ray absorption spectroscopy (XAS) and in situ EXAFS techniques.
The XAS measurements revealed that the samples contained redox-active Fe with oxidation states higher than three and showed distorted [MO6] octahedra. The in situ EXAFS data showed a substantial cobalt reoxidation behavior and a shorter Fe-O bond length. The quasi in situ XAS measurements of the samples before the redox peak and during OER demonstrated strong interactions between iron and cobalt, leading to a redox activity of the iron centers with oxidation states up to 3.2.
The results of this study are significant as they demonstrate the high oxidation states of iron in cobalt-iron oxyhydroxides, which improves their electrocatalytic properties. These findings could have implications for a wide range of applications in energy conversion and storage.
In Situ Detection of Iron in Oxidation States ≥ IV in Cobalt-Iron Oxyhydroxide Reconstructed during Oxygen Evolution Reaction
Reith; Hausmann; Mebs; Mondal; Dau; Driess; Menezes
Full-text link: https://doi.org/10.1002/aenm.202203886
What this paper is about
- The formed helices are “left-handed” or “right-handed” plane.
- Supporting Information shows the pink single crystals from Fe 0.55 Co 0.45 BPO with a smooth surface and hexagonal bipyramidal shape typical for all Fe 1x Co x BPO samples.
What you can learn
- Quasi in situ XAS measurements on these samples show that the Co. O bond distances and cobalt oxidation states are comparable to those of bulk-active amorphous cobalt catalysts, extrapolations of all surface di-oxo cobalt sites under OER conditions, and the average bond length of Co 4 O 4 cubane model compounds under OER conditions, showing that these samples behave comparable to an all-surface structure, due to their electrolyte-penetrability.
- Quasi in situ XAS of the four different cobalt oxyhydroxides at a potential before their redox peak and during OER revealed a substantial cobalt redox behavior with an oxidation state of around 2.9 below the redox peak and 3.2 at OER conditions.
- Consistent with the high oxidation state of iron, we find an in situ Fe.
- The quasi in situ XAS measurements of the four different cobalt oxyhydroxides before the redox peak and during OER demonstrate strong interactions between iron and cobalt correlated to an anodic redox peak shift, without a decrease of the redox activity through the addition of iron.
- Moreover, in contrast to various previous investigations, a redox activity of the iron centers can be observed with oxidation states up to 3.2.
- O bond distance is consistent with the presence of a -oxyl radical.
What is the paper about?
The paper is about the electrocatalytic properties of cobalt-iron oxyhydroxides (Fe1-xCoxBPO) synthesized by mild hydrothermal and high-energy ball milling methods. The properties were investigated using electrochemical and analytical techniques, including quasi in situ X-ray absorption spectroscopy (XAS).
What can we learn from this paper?
The paper shows that the cobalt oxidation states and Co-O bond distances of these samples are comparable to bulk-active amorphous cobalt catalysts. The XAS measurements reveal strong interactions between iron and cobalt and a redox activity of the iron centers up to 3.2 oxidation states.
What techniques were used to study the electrocatalytic properties?
The electrocatalytic properties were studied using quasi in situ X-ray absorption spectroscopy (XAS), electrochemical techniques, and analytical techniques.
What was the result of the quasi in situ XAS measurements of the cobalt oxyhydroxides?
The XAS measurements revealed a substantial cobalt redox behavior with an oxidation state of around 2.9 below the redox peak and 3.2 at OER conditions. The iron centers showed a redox activity of up to 3.2 oxidation states, and strong interactions between iron and cobalt were observed.
What is the shape of the single crystals of Fe0.55Co0.45BPO in Supporting Information?
The single crystals of Fe0.55Co0.45BPO in Supporting Information are hexagonal bipyramidal with a smooth surface.