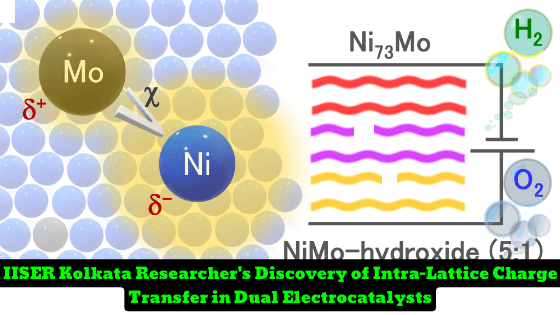
Researchers at the Indian Institute of Science Education and Research (IISER) Kolkata have made an exciting discovery in the field of electrocatalysis. They have identified an unorthodox intra-lattice ‘inverse’ charge transfer from molybdenum (Mo) to nickel (Ni) in two systems – Ni73Mo alloy electrodeposited on copper nanowires and NiMo-hydroxide (Ni:Mo = 5:1) on Ni foam. The discovery is expected to have significant implications for fine-tuning the electronic structure of active centers in electrocatalysts.
Details of the Discovery
The researchers used X-ray absorption fine structure studies and X-ray photoelectron spectroscopy to decipher the inverse charge transfer effect. They found that the undercoordinated Mo-center pushes the Mo 4d-orbitals close to the Fermi energy in the valence band region, while Ni 3d-orbitals lie in the conduction band. Electrons are donated from the electron-rich Mo-center to the electron-poor Ni-center, causing the Mo-center to become positively charged and vice versa, resulting in the reverse charge distribution in Ni73Mo.
Outcomes of the Discovery
The reverse charge distribution in Ni73Mo accelerates the electrochemical hydrogen evolution reaction in both alkaline and acidic media. The electrocatalyst has a 0.35 and 0.07 s-1 turnover frequency at -33 ± 10 and -54 ± 8 mV versus reversible hydrogen electrode, respectively. The corresponding mass activities are 10.5 ± 2 and 2.9 ± 0.3 A g-1 at 100, and 54 mV overpotential, respectively. The researchers also found that anodic potential oxidizes the Ni-center of NiMo-hydroxide for alkaline water oxidation, achieving a 0.43 O2 s-1 turnover frequency at 290 mV overpotential. The homologous couple of NiMo-hydroxide and Ni73Mo also achieves water and urea splitting with cell voltages of 1.48 ± 0.02 and 1.32 ± 0.02 V, respectively, at 10 mA cm-2.
The discovery of an unorthodox intra-lattice ‘inverse’ charge transfer in electrocatalysts is a significant step forward in fine-tuning the electronic structure of active centers. The IISER Kolkata researchers’ findings will pave the way for the development of more efficient and durable electrocatalysts for various applications, including water splitting, urea splitting, and hydrogen evolution.
Inverse ‘Intra-Lattice’ Charge Transfer in Nickel-Molybdenum Dual Electrocatalysts Regulated by Under-coordinating the Molybdenum Center
Chemical-Science; Bothra; Dutta; Maji; Mura; Kumar; Chaudhary; Rajput; Kumar; Pati; Parvin; Bothra; Dutta; Maji; Mura; Kumar; Chaudhary; Rajput; Kumar; Pati; Bhattacharyya 2023
Full-text link: https://doi.org/10.1039/D2SC04617B
What this paper is about
- IISER Kolkata Researcher have coined the term “intra-lattice” for the charge transfer occurring within the same lattice, in order to differentiate it from the “inter-lattice” charge transfer processes that occur between two phases across the heterogeneous interfaces, or from the substrate to the catalyst under the influence of applied potential.
- Ni has a lower free energy of adsorption of OER intermediates, as well as the nominal interaction between O 2p of OH radicals and Ni 3d orbitals facilitates the desorption of H 2 from Ni center.
- Hall analysis shows the increase in lattice strain from 1.9 -3 in Ni-100-60m to 4 -3 after the incorporation of Mo in Ni 73 Mo.
What you can learn
- Gibbs free energy for each adsorbate was calculated by equation: = + where E is the adsorption energy.
- Hence, we have considered U = 0 V potential for all the steps.
- As the vibration entropy of the adsorbed H* is small, the entropy of adsorption of H 2 is considered as S H ~ S H2 where S H2 is the entropy of H 2 in the gas phase under standard conditions.
Basics Q&A related to this research
What is intermetallic charge transfer?
Intermetallic charge transfer is the transfer of charge between two different metals or intermetallic compounds that results in the fine-tuning of electronic structure of the active centers in electrocatalysts.
What is Pauling electronegativity?
Pauling electronegativity is a concept that explains the ability of an atom to attract shared electrons towards itself in a chemical bond.
What is Ni73Mo alloy electrodeposited on Cu nanowires?
Ni73Mo alloy electrodeposited on Cu nanowires is a type of dual electrocatalyst used for accelerating the electrochemical hydrogen evolution reaction in alkaline and acidic media.
What is NiMo-hydroxide?
NiMo-hydroxide is another type of dual electrocatalyst used for alkaline water oxidation.
What is X-ray absorption fine structure?
X-ray absorption fine structure is a spectroscopic technique that provides information on the local atomic environment around a specific element in a material.
What is X-ray photoelectron spectroscopy?
X-ray photoelectron spectroscopy is a technique that provides information about the chemical composition and electronic structure of materials.
What is Bader charge?
Bader charge is a quantum chemical method that is used to analyze the charge distribution within a material.
What is projected density of state?
Projected density of state is a theoretical concept that is used to calculate the electronic structure of materials.
What is electrochemical hydrogen evolution reaction?
Electrochemical hydrogen evolution reaction is a process of generating hydrogen gas from water using an electrocatalyst.
What is meant by alkaline and acidic?
Alkaline and acidic are two types of solutions with different pH values. Alkaline solutions have a pH greater than 7 while acidic solutions have a pH less than 7.
What is turnover frequency?
Turnover frequency is a measure of the number of times a catalyst can convert a reactant to a product per unit time.
What is reversible hydrogen electrode?
Reversible hydrogen electrode is a reference electrode used to measure the electrochemical potential of hydrogen in a solution.
What are mass activities?
Mass activities are the measure of the efficiency of a catalyst in converting reactants to products.
What is alkaline water oxidation?
Alkaline water oxidation is a process of oxidizing water using a catalyst in an alkaline solution.
What is urea splitting?
Urea splitting is a process of breaking down urea into ammonia and carbon dioxide using a catalyst.
What are cell voltages?
Cell voltages are the measure of the potential difference between the two electrodes in an electrochemical cell.
What is lattice strain?
Lattice strain is the distortion in the crystal structure of a material due to the presence of impurities or defects.
What is Gibbs free energy?
Gibbs free energy is a thermodynamic concept that determines the spontaneity and direction of a chemical reaction.
What is adsorption energy?
Adsorption energy is the energy released or absorbed when a molecule or atom adsorbs onto a surface.
What is vibration entropy?
Vibration entropy is the measure of the degree of freedom of the atoms in a molecule due to their vibrational motion.
What is entropy of adsorption?
ntropy of adsorption is the measure of the degree of freedom of the adsorbate when it adsorbs onto a surface.